Abstract
Background
Prostate biopsy (PB) is the gold standard for verifying the diagnosis of prostate cancer in men with clinical symptoms. Transrectal ultrasound (TRUS)-guided PB is the most common method for diagnosis; however, it has a few adverse effects. Mild consequences like bleeding and pain are prevalent but temporary. Since the relationship between erectile dysfunction and lower urinary tract symptoms (LUTS) and TRUS-guided PB is inconsistent in the literature, we aimed to conduct a study on these two consequences on males within 1 month following TRUS-guided PB.
Methods
Patients with a PSA ≥ 4 ml/ng who were determined to undergoTRUS-guided PB were enrolled in this prospective study. Patients' urinary symptoms and erectile function were evaluated using the International Prostate Symptom Score (IPSS) and International Index of Erectile Function-5 (IIEF-5) questionnaires the day before and 1 month after PB. Also, using uroflowmetry, the patients' urinary peak flow rate (Qmax) was recorded. SPSS version 18 was used to compare and analyze variables.
Results
The mean age of the participants was 67.47 ± 9.38 years. Before the PB, the IIEF-5 score was 20.19 ± 7.24, and after the PB, it was 20.25 ± 7.24 (p = 0.865). The Qmax level rose from 7.35 ± 2.15 to 7.74 ± 2 ml/s (p = 0.07). After TRUS-guided PB, the average IPSS score reduced from 11.48 ± 9.93 to 9.88 ± 8.22 which was statistically significant (p < 0.001).
Conclusions
This study indicated that TRUS-guided PB had no negative impact on erectile function or LUTS in participants and may even relieve urinary symptoms to some extent. Overally, TRUS-guided PB appears to be a safe strategy for evaluating prostate cancer suspects.
Similar content being viewed by others
1 Background
Prostate cancer is by far the most common malignant tumor in elderly males [1]. The digital rectal examination (DRE), the prostate-specific antigen (PSA) blood test, and prostate biopsy (PB) are diagnostic tools for screening for prostate cancer at an early, treatable stage [2]. A PB is the gold standard approach for confirming the diagnosis of prostate cancer in men with symptoms of the disease [3]. The systematic 12-core transrectal prostate biopsy guided by transrectal ultrasound (TRUS) has become a well-established, routinely performed approach for detecting prostate cancer [4]. Although transperineal prostate biopsy is associated with fewer complications than the transrectal biopsy, the technique's adoption has been gradual due to the perception that general or spinal anesthesia is required, and transrectal biopsy remains the most prevalent technique for prostate biopsy [5]. Moreover, methods such as MRI-targeted biopsy are already utilized for this purpose and MRI is more accurate than TRUS. However, TRUS is less expensive, more accessible, and nearly as accurate as MRI, making it the preferable method for prostate biopsy [6, 7]. Generally, TRUS-guided PB is well tolerated and carries a minimal risk of serious consequences. Nevertheless, mild complications such as pain and bleeding are common but often transient [8]. Regardless of the biopsy method, mild, self-limiting bleeding (hematuria, hematospermia) is the most common post-PB consequence [9]. The most serious consequences consist of sepsis and severe hematuria [10].
An erection in the penile region results from a series of complex neurovascular, humoral, and psychological events [11]. Erectile dysfunction (ED) is characterized as the chronic inability to attain and keep an erection enough for satisfying sexual performance [12]. Several factors, such as anesthesia, age, mental stress, and injury to the neurovascular bundles, may influence or impair normal erectile function following PB [11]. LUTS are symptoms caused by diseases and conditions influencing the bladder and urethra, such as urinary incontinence (e.g., stress, urge, and mixed urinary incontinence); storage/overactive bladder symptoms (e.g., urgency, frequency, and nocturia, with or without incontinence); voiding (e.g., urinary retention, hesitancy, straining to void, slow or interrupted stream); and postmicturition (e.g., terminal dribbling) [13].
LUTS and ED are among the adverse symptoms observed following a PB [14]. The evidence for ED and LUTS following a TRUS-guided PB is contradictory, though most studies suggest that these two complications are only temporary after a TRUS-guided PB. This study's objective is to examine the effects of TRUS-guided PB on ED and LUTS.
2 Methods
2.1 Study design and sampling
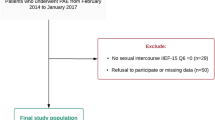
The research design was a prospective study. All patients referred to the urology clinic of Razi Hospital in Rasht who had a PSA above 4 ml/ng and were determined to undergo PB under TRUS guidance were evaluated over 1 year. As background and intervening variables, the patients' age, history of surgery and anesthesia, PSA level, comorbid diseases, history of urinary tract infection, and pharmacological treatment for lower urinary tract symptoms were questioned after gaining their consent. Patients with a penile prosthesis, a biopsy within the last 6 weeks, a history of lower urinary tract disease (bladder stones, neurogenic bladder, bladder cancer, and urethral stricture), urinary retention, or active urinary infection were excluded from the study.
2.2 IPSS and IIEF-5 questionnaires
All patients underwent TRUS-guided PB with the standard twelve cores at the outpatient urology clinic. No new medications were administered to the patients after the biopsy. The patients' urinary symptoms and sexual function were assessed using the International Prostate Symptom Score (IPSS) and International Index of Erectile Function-5 (IIEF-5) questionnaires the day before and 1 month afterward the biopsy. The IPSS questionnaire consists of seven questions about incomplete bladder emptying, frequency, urinary hesitancy, urgency, weak urinary flow, straining, and nocturia, and each item has a scoring range of 0- 5. The total number of points is 35. A score between 0 and 7 indicates a mild urinary disorder, 8–19 a moderate condition, and 20–35 a severe condition. The five-item IIEF-5 questionnaire comprises questions concerning achieving an erection during sex, maintaining it till the end of the intercourse, experiencing satisfaction from the intercourse, and the number of times the person had sex in the previous month. There are five classifications for the severity of sexual dysfunction: 0–7 severe dysfunction, 8–11 moderate, 12–16 mild to moderate, 17–21 mild, and 22–25 no dysfunction.
2.3 Uroflowmetry
Uroflowmetry was performed on patients the day before and 1 month after the biopsy to assess any changes in urinary flow. Uroflowmetry is a noninvasive and low-cost screening test for individuals with urination disorders that provides information on storage and voiding symptoms. The recorded pattern of urine flow is sometimes used for potential diagnosis. The maximum urinary flow rate, total urine volume excreted, average urinary flow, and residual urine after emptying can also be determined from the data collected. Patients' maximum flow rates (Qmax) were recorded and compared before and after the biopsy.
2.4 Statistical analysis
The information was organized using descriptive statistics, tables, and graphs after the data had been collected, and the relative frequency and 95% confidence interval (95% CI) were used to calculate the average level of lower urinary symptoms and sexual performance. The IPSS and IIEF-5 scores and Qmax levels were compared before and after biopsy using the paired T-test in the case of normal distribution and the nonparametric Wilcoxon test in the non-normal distribution. One-way analysis of variance (ANOVA) and the Kruskal–Wallis test will be utilized to compare the changes based on background and confounding variables. The significance level for all tests was set at 0.05, and SPSS 18 was used for statistical analysis.
3 Results
This study evaluated the research variables of 70 patients who received TRUS-guided prostate biopsies. The participants had an average age of 67.47 ± 9.38 years. This research evaluated the complete information of 63 samples in terms of Qmax, 63 samples in terms of IPSS score, and 55 samples in terms of IIEF-5 score before and after TRUS-guided PB. The results indicated that the Qmax score before and after the biopsy followed a normal distribution, whereas the IPSS and IIEF scores did not; therefore, the paired T-test was used to compare the Qmax levels, while the Wilcoxon test was utilized to compare the IPSS and IIEF-5 scores.
Table 1 compares the average IIEF-5 and IPSS before and after the TRUS-guided PB. Based on the data, the IIEF-5 score was 20.19 ± 7.24 before the PB and 20.25 ± 7.24 after the PB. Using the Wilcoxon test, there was no statistically significant difference in the average IIEF-5 before and after the biopsy (p = 0.865). The mean IPSS score after TRUS-guided PB decreased by around 2 points from 11.48 ± 9.93 to 9.88 ± 8.22, and this difference was statistically significant using the Wilcoxon test (p < 0. 001).
According to Table 2, despite an increase of 0.4 ml/s in the Qmax level from 7.35 ± 2.15 to 7.74 ± 2 ml/s, this difference was not statistically significant (p = 0.07) using the paired T-test. Figure 1 illustrates the comparison between the 95% confidence intervals of all three variables before and after biopsy.
Table 3 displays the changes in IPSS, IIEF-5 scores, and Qmax before and after TRUS-guided PB based on age and PSA level variables using Spearman's rho correlation coefficient. There was no statistically significant association between these three variables and age or PSA level.
The nonparametric Mann–Whitney test was employed to compare the changes in the aforementioned scores with other background and intervening variables under consideration. There was no correlation between the use of terazosin, finasteride, tamsulosin, or prazosin, or a history of herniorrhaphy, hemorrhoidectomy, varicocelectomy, cholecystectomy, hypothyroidism, hypertension, ischemic heart disease, chronic kidney disease, diabetes, chronic lung disease, or open heart surgery, and these score changes in terms of all three variables (p > 0.05).
4 Discussion
In this study, the participants' average age was 67.47 years. The score of the IIEF-5 questionnaire was 20.19 before the PB and increased to 20.25 1 month after the procedure (p = 0.865). The Qmax level increased from 7.35 to 7.74 ml/s, with a significance level of p = 0.07. Also, following TRUS-guided PB, the patients' average IPSS score dropped from 11.489 to 9.88, and this difference was significant (p < 0.001).
In males, ED is a frequent and significant quality of life concern, and those candidates for prostate biopsies are particularly concerned about this issue. The pathophysiology of ED is not yet fully understood. Direct structural damage to the neurovascular bundle surrounding the prostate or pressure on nerves caused by a hematoma or edema after biopsy might cause ED [15].
While some studies suggest that a PB has no effects on erectile dysfunction, others suggest that it can induce ED in the short and long term. In a study by Kamali et al., there was a statistically significant drop from a mean score of 23 on the IIEF-5 questionnaire 1 month prior to the biopsy to a score of 18 1 month after the procedure. In a recent study by Tuncel et al., the mean IIEF-5 score was 20.8 prior to biopsies and 17.4 3 months afterward; this difference was statistically significant [16]. In the study of Murray et al., 1 week after the biopsy, the IIEF-5 score was significantly lower than before the biopsy (18.2 vs. 15.5). This remained considerably decreased after 4 (17.3) and 12 weeks (16.9) following the biopsy [17]. In contrast, the current study discovered that TRUS-guided PB had no significant effect on erectile function. Similarly, in a study by García Rojo et al., there were no significant differences between pre-biopsy and post-biopsy IEEF-5 scores at 3 and 6 months, and neither the number of biopsy cores nor the number of previous biopsies influenced IEEF-5 scores [18]. The majority of studies, however, suggest that TRUS-guided PB only has a temporary impact on erectile function [19,20,21]. Consequently, according to the studies and the postulated mechanism for ED, the occurrence rate of ED can be attributed to the accuracy of the procedure.
LUTS can be unpleasant and socially isolating since the fear of urine leakage can lower self-esteem. Higher rates of mental health problems and diminished quality of life have been linked to LUTS [22]. In a study by Borghe et al., the prevalence of LUTS following TRUS-guided PB was found to be 25% [9]. However, many of these patients have previously had LUTS due to their prostate hypertrophy.
Various research has yielded contradictory findings about LUTS. Multiple studies have indicated a correlation between PB and transient LUTS [15, 23]. The study by Tobias et al. revealed that the IPSS score increased significantly in the first week after the biopsy compared to before the biopsy but did not increase significantly in the 4th and 12th weeks compared to the baseline score [23]. On the contrary, some studies indicate that prostate biopsy does not raise the risk of urinary symptoms. In a study performed by Helfand et al. utilizing the American Urological Association Symptom Index (AUA-SI) questionnaire, no correlation was seen between LUTS and TRUS-guided PB [24]. In our study, the mean IPSS score was reduced by around 2 points which was a significant decrease. In a study conducted by Murray et al., the mean IPSS before TRUS-guided PB was 10.2, while the mean IPSS at 1 week, 4 weeks, and 3 months after the procedure decreased to 9.5, 9.4, and 9.3, representing statistically significant changes at 1 week and 4 weeks after PB [17]. Similar to our investigation, this study demonstrated evidence in favor of LUTS improvement following biopsy.
This decrease in the score and relative improvement in the urinary symptoms of patients may have multiple causes. Firstly, this demonstrates that the performed biopsies did not result in any consequences due to urethral stricture. Furthermore, the reduction in lower urinary tract symptoms may be attributable to the psychological impact of the patients' assurance that they would not develop cancer. Urinary symptoms can be exacerbated by stress, in this study stress related to having prostate cancer.Chronic stress results in the release of cytokines and chemokines that promote inflammation. Pro-inflammatory cytokines alter the regulation of fluid overload pathways in the brain and spinal cord. These cytokines can also disrupt the bladder's function by directly promoting detrusor hypertrophy and afferent nerve hypersensitivity and consequently cause LUTS [25,26,27]. One-fourth of males seeking a urologic consultation exhibit concurrent depression, anxiety, or stress symptoms [26]. In our study, the urinary symptoms may have been exacerbated by the fear of having prostate cancer prior to the biopsy; However, since this anxiety has been alleviated for most patients whose test results were negative, the urinary symptoms may have been reduced as a result. Moreover, the multiple biopsies done may have decreased the prostate's volume and, as a result, decreased the pressure on the urethra and relieved the patients' symptoms. Although the decreased volume of the prostate at the time of biopsy may be insignificant, there is still a possibility of reducing the strain on the urethra.
Since the IPSS score is a subjective evaluation, we opted to examine LUTS before and after biopsy using objective techniques, such as the Qmax parameter. Prior to the biopsy, the average Q max level was 7.35 ml/s, but afterward, it rose to 7.74 ml/s. Although this increase was not statistically significant, its accordance with the decrease in the IPSS score suggests that PB has a beneficial influence on LUTS. However, to determine the positive association between PB and LUTS, uroflowmetry with larger sample size is required.
This study had some strengths and limitations to be noted. The use of uroflowmetry to confirm the changes in urinary symptoms was one of the study’s strengths. One of the limitations of our study was that the follow-up was conducted only 1 month after the biopsy and not at longer intervals, which could have had an impact on the significance of the changes in the variables. Moreover, regarding the psychological influence of biopsy results on urine symptoms, it was unclear if patients with reduction in urinary symptoms had positive or negative biopsy results, and whether the reduction in their urinary symptoms was followed by a decrease in stress or not. It is suggested that additional studies be carried out in this field in the future. Additionally, it is advised that changes in prostate volume be evaluated prior to and following the biopsies to determine whether the volume reduction of the prostate caused by biopsy is significant to improve urinary symptoms.
5 Conclusions
This study demonstrated that PB using TRUS had no adverse effects on erectile function and urinary symptoms in patients and may even relieve urinary symptoms to some extent. However, since uroflowmetry did not reveal a significant increase in Qmax, it is required to perform further research about urinary flow alterations following PB with larger sample sizes and longer follow-up periods. In general, TRUS-guided PB appears to be a safe method for evaluating patients suspected of prostate cancer.
Availability of data and materials
Data will be provided by the corresponding author on request.
Abbreviations
- DRE:
-
Digital rectal examination
- PSA:
-
Prostate-specific antigen
- PB:
-
Prostate biopsy
- TRUS:
-
Transrectal ultrasound
- ED:
-
Erectile dysfunction
- IPSS:
-
International Prostate Symptom Score
- IIEF-5:
-
Index of erectile function-5
- Qmax:
-
Maximum flow rates
- ANOVA:
-
Analysis of variance
- AUA-SI:
-
American Urological Association Symptom Index
References
Pinkhasov GI, Lin YK, Palmerola R, Smith P, Mahon F, Kaag MG, Dagen JE, Harpster LE, Reese CT, Raman JD (2012) Complications following prostate needle biopsy requiring hospital admission or emergency department visits—experience from 1000 consecutive cases. BJU Int 110(3):369–374. https://doi.org/10.1111/j.1464-410X.2011.10926.x
Ilic D, Neuberger MM, Djulbegovic M, Dahm P (2013) Screening for prostate cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004720.pub3
Huang H, Wang W, Lin T, Zhang Q, Zhao X, Lian H, Guo H (2016) Comparison of the complications of traditional 12 cores transrectal prostate biopsy with image fusion guided transperineal prostate biopsy. BMC Urol 16(1):68. https://doi.org/10.1186/s12894-016-0185-z
Hara R, Jo Y, Fujii T, Kondo N, Yokoyoma T, Miyaji Y, Nagai A (2008) Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology 71(2):191–195. https://doi.org/10.1016/j.urology.2007.09.029
Thomson A, Li M, Grummet J, Sengupta S (2020) Transperineal prostate biopsy: a review of technique. Transl Androl Urol 9(6):3009
Lee JS, Chung BH (2007) Transrectal ultrasound versus magnetic resonance imaging in the estimation of prostate volume as compared with radical prostatectomy specimens. Urol Int 78(4):323–327. https://doi.org/10.1159/000100836
Eklund M, Jäderling F, Discacciati A, Bergman M, Annerstedt M, Aly M, Glaessgen A, Carlsson S, Grönberg H, Nordström T (2021) MRI-targeted or standard biopsy in prostate cancer screening. N Engl J Med 385(10):908–920
Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, Rosario DJ, Scattoni V, Lotan Y (2013) Systematic review of complications of prostate biopsy. Eur Urol 64(6):876–892. https://doi.org/10.1016/j.eururo.2013.05.049
Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, Weidner W, Loeb S (2017) Complications after systematic, random, and image-guided prostate biopsy. Eur Urol 71(3):353–365. https://doi.org/10.1016/j.eururo.2016.08.004
Huang H, Wang W, Lin T, Zhang Q, Zhao X, Lian H, Guo H (2016) Comparison of the complications of traditional 12 cores transrectal prostate biopsy with image fusion guided transperineal prostate biopsy. BMC Urol 16(1):1–6
Helfand BT (2015) Temporary erectile dysfunction after prostate biopsy. BJU Int 116(2):164. https://doi.org/10.1111/bju.13022
De Nunzio C, Roehrborn CG, Andersson K-E, McVary KT (2017) Erectile dysfunction and lower urinary tract symptoms. Eur Urol Focus 3(4):352–363. https://doi.org/10.1016/j.euf.2017.11.004
Zhang AY, Xu X (2018) Prevalence, burden, and treatment of lower urinary tract symptoms in men aged 50 and older: a systematic review of the literature. SAGE Open Nurs 4:2377960818811773. https://doi.org/10.1177/2377960818811773
Kamali K, Nabizadeh M, Ameli M, Emami M, Mahvari-Habibabadi M, Amirpoor M (2019) Impact of prostate needle biopsy on erectile function: a prospective study. Urologia 86(3):145–147. https://doi.org/10.1177/0391560319834488
Glaser AP, Novakovic K, Helfand BT (2012) The impact of prostate biopsy on urinary symptoms, erectile function, and anxiety. Curr Urol Rep 13(6):447–454. https://doi.org/10.1007/s11934-012-0277-6
Tuncel A, Toprak U, Balci M, Koseoglu E, Aksoy Y, Karademir A, Atan A (2014) Impact of transrectal prostate needle biopsy on erectile function: results of power Doppler ultrasonography of the prostate. Kaohsiung J Med Sci 30(4):194–199. https://doi.org/10.1016/j.kjms.2013.11.004
Murray KS, Bailey J, Zuk K, Lopez-Corona E, Thrasher JB (2015) A prospective study of erectile function after transrectal ultrasonography-guided prostate biopsy. BJU Int 116(2):190–195. https://doi.org/10.1111/bju.13002
García Rojo E, García Gómez B, González Padilla DA, Abad López P, García González L, Rodríguez Antolín A, Romero Otero J (2019) Assessment of the influence of transrectal and transperineal prostate biopsies on erectile function: a prospective observational single-center study. Int J Urol 26(11):1054–1058. https://doi.org/10.1111/iju.14088
Whitson JM, Murray KS, Thrasher JB (2016) Prostate biopsy is associated with an increased risk of erectile dysfunction. J Urol 196(1):21–23. https://doi.org/10.1016/j.juro.2016.04.002
Mehta A, Kim WC, Aswad KG, Brunckhorst O, Ahmed HU, Ahmed K (2021) Erectile function post prostate biopsy: a systematic review and meta-analysis. Urology 155:1–8. https://doi.org/10.1016/j.urology.2021.01.035
Fainberg J, Gaffney CD, Pierce H, Aboukhshaba A, Chughtai B, Christos P, Kashanian JA (2021) Erectile dysfunction is a transient complication of prostate biopsy: a systematic review and meta-analysis. J Urol 205(3):664–670. https://doi.org/10.1097/ju.0000000000001398
Yoo TK, Lee K-S, Sumarsono B, Kim S-T, Kim H-J, Lee H-C, Kim S-H (2018) The prevalence of lower urinary tract symptoms in population aged 40 years or over, in South Korea. Investig Clin Urol 59(3):166–176
Klein T, Palisaar RJ, Holz A, Brock M, Noldus J, Hinkel A (2010) The impact of prostate biopsy and periprostatic nerve block on erectile and voiding function: a prospective study. J Urol 184(4):1447–1452
Helfand BT, Glaser AP, Rimar K, Zargaroff S, Hedges J, McGuire BB, Catalona WJ, McVary KT (2013) Prostate cancer diagnosis is associated with an increased risk of erectile dysfunction after prostate biopsy. BJU Int 111(1):38–43. https://doi.org/10.1111/j.1464-410X.2012.11268.x
Chess-Williams R, McDermott C, Sellers DJ, West EG, Mills KA (2021) Chronic psychological stress and lower urinary tract symptoms. Low Urin Tract Symptoms 13(4):414–424. https://doi.org/10.1111/luts.12395
Vartolomei L, Cotruș A, Tătaru SO, Vartolomei MD, Man A, Ferro M, Stanciu C, Sin AI, Shariat SF (2022) Lower urinary tract symptoms are associated with clinically relevant depression, anxiety, and stress symptoms. Aging Male 25(1):55–59. https://doi.org/10.1080/13685538.2022.2040981
Martin S, Vincent A, Taylor AW, Atlantis E, Jenkins A, Januszewski A, O’Loughlin P, Wittert G (2015) Lower urinary tract symptoms, depression, anxiety and systemic inflammatory factors in men: a population-based cohort study. PLoS ONE 10(10):e0137903. https://doi.org/10.1371/journal.pone.0137903
Acknowledgements
None to declare.
Funding
This research did not receive any funding.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by HN. Methodology was done by HN and AA. Validation was done by AA and SAA. Formal analysis was done by ZM and AA. Investigation was done by EK and MH. Data curation was done by MH. Original draft preparation was done by RS and ZM. Review and editing were done by ZM and SAA. Visualization was done by SAA. Supervision was done by HN. Project administration was done by RS. All authors in the article have made significant contributions, and all authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of the Urology Research Center of Guilan University of Medical Sciences granted legal and ethical approval. The patients gave written informed consent to participate in the study.
Consent for publication
The patients have given us informed consent for publication.
Competing interests
The authors disclose no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Nasseh, H., Asgari, S.A., Sarmadian, R. et al. The effect of transrectal ultrasound-guided prostate biopsy on erectile function and lower urinary tract symptoms: a prospective study. Afr J Urol 29, 14 (2023). https://doi.org/10.1186/s12301-023-00345-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-023-00345-7