Abstract
Objective
This study aimed to evaluate the association of single nucleotide polymorphisms (SNPs) of vitamin D metabolic pathway genes with susceptibility to pulmonary tuberculosis (PTB).
Methods
Nine hundred seventy-nine patients (490 PTB cases and 489 healthy controls) were included in this study. Seventeen SNPs of vitamin D metabolic pathway genes, including CYP24A1, CYP27A1, CYP27B1, CYP2R1, GC, and DHCR7, were genotyped with improved multiple ligase detection reaction (iMLDR).
Results
The GC rs3733359 GA, rs16847024 CT genotypes were significantly associated with the reduced risk of PTB, and the rs3733359 A, rs16847024 T alleles were also associated with the decreased PTB susceptibility. The GT genotype of GC rs4588 variant was significantly higher in patients with PTB when compared to controls. Moreover, the increased risk of rs3733359 and rs16847024 variants, and a decreased risk of rs4588, were found under the dominant mode among the PTB patients. However, there was no significant relationship of CYP24A1, CYP27A1, CYP27B1, CYP2R1, and DHCR7 polymorphisms with the risk of PTB. In CYP27A1, the rs17470271 T and rs933994 T alleles were significantly associated with leukopenia, drug resistance in the PTB patients, respectively. In GC gene, the rs7041 and rs3733359 variants were found to be associated with pulmonary infection, fever in the PTB patients, respectively. The increased frequency of rs16847024 TT genotype was found in the PTB patients with fever and drug-induced liver damage. DHCR7 rs12785878 TT genotype, and T allele frequencies were both significantly associated with pulmonary infection in the PTB patients. The haplotype analysis showed that CYP24A1 TACT, CYP2R1 GGCT, GGAT, GC AATG haplotypes were related to PTB susceptibility.
Conclusion
Our study suggested that GC SNPs were associated with the genetic background of PTB. CYP27A1, GC, and DHCR7 genetic variations might contribute to several clinical phenotypes of PTB in Chinese.
Similar content being viewed by others
Introduction
Pulmonary tuberculosis (PTB), which is caused by the human pathogen Mycobacterium tuberculosis (MTB), continues to be the leading cause of morbidity and mortality worldwide [1]. The latest World Health Organization (WHO) figures showed that there were about 10.0 million new TB patients globally in 2018. Of them, 0.87 million cases were from China [2]. Currently, PTB poses a major public health challenge and is a significant economic burden in Asian region; hence, it is important to identify factors that increase disease susceptibility in order to provide evidence for effective control strategies. Cell-mediated immunity is essential for inhibiting MTB infection, regulating the first defense against MTB, and has an important role in the development of PTB [3]. In addition, previous studies had shown that only 10% of these people infected with MTB finally developed active PTB, and the identical twins were twice as likely to progress to this disease as fraternal twins [4, 5]. These facts supported the viewpoint that susceptibility to PTB upon MTB infection was affected by the host genetic and environmental factors. Variation of many genes had been identified to be associated with genetic susceptibility to PTB in previous studies [6,7,8].
The existing evidence showed that the vitamin D metabolic pathway might be implicated to the pathogenesis of PTB. Vitamin D deficiency was much more common in patients with PTB, and the serum vitamin D expression was negatively related to disease severity [9, 10]. Another study found that there was ethnic difference in the 25-hydroxyvitamin D (25(OH)D) level among the new TB cases, and further studies confirmed that vitamin D deficiency resulted in reduced immunity against bacteria, and increased the risk of TB [11,12,13]. 1-25-dihydroxyvitamin D3 (1,25(OH)2D3), known as the active form of vitamin D, is a key hormone that regulated the activity of different defense and immune cells, including epithelial, lymphocytes, macrophages, and monocytes cells [14]. 1,25(OH)2D3 has also been reported to increase innate immunity through enhancing the antimicrobial peptides expression, and promoting the autophagy of infected cells, thereby restraining the MTB growth in macrophages cells [15]. Immune modulation by vitamin D included enhancing innate immune response, attenuating and stimulating Th1 and Th2 cell proliferation, respectively, and hypovitaminosis D was associated with autoimmune diseases, Alzheimer’s disease, etc. [16, 17]. Hence, the influence of the individual variations in vitamin D metabolism on the immune responses, patients should not be neglected in patients with PTB.
Many genes associated with vitamin D metabolic pathway were involved in the host susceptibility to persistent TB infection in which vitamin D receptor (VDR) had been well studied [18,19,20]. Vitamin D exerted its biological functions through binding with VDR; hence, VDR variant might contribute to the TB development by resulting in diminished function of vitamin D. The study by Hu et al. demonstrated a significant association between VDR rs11574143, rs11168287, rs11574079 polymorphisms and PTB susceptibility in the Chinese population [21]. Besides VDR, other genes involved in vitamin D pathway, including CYP24A1, CYP27A1, CYP27B1, CYP2R1, GC, DHCR7, might also be associated with TB susceptibility [22]. It is very meaningful to study the influence of genetic variation of multiple genes in the context of vitamin D metabolic pathway, rather than a single gene, in the development of PTB. However, there were no studies to analyze the relationship of the collective vitamin D metabolic pathway genes and PTB susceptibility in a Chinese population. Thus, this study was designed to evaluate the associations of single nucleotide polymorphisms (SNPs) of vitamin D pathway genes (CYP24A1, CYP27A1, CYP27B1, CYP2R1, GC, DHCR7) with PTB susceptibility in a Chinese population.
Materials and methods
Study participants
In this case-control study, we recruited 500 patients with PTB from the Department of Tuberculosis at Anhui Chest Hospital during the period of April to October 2019. The PTB patients were diagnosed by a specialist according to the following criteria: suspicious clinical symptoms, chest radiography, sputum and/or bronchoalveolar lavage fluid MTB culture, microscopy for acid fast bacilli (AFB), and effect of anti-TB treatment. We excluded PTB patients with HIV positive, hepatitis, malignant tumor, and immune-compromised conditions. We enrolled a random sample of 500 unrelated healthy individuals without a history of TB, malignant tumor, and HIV, from health examine center in the same area to serve as controls. All controls needed to be asymptomatic with negative sputum smear and culture, and normal chest radiograph.
Our study was approved by the Ethics Committee of Anhui Chest Hospital (K2020-005). We collected peripheral blood samples and other information from the study participants after obtaining an informed consent from each participant. The information included age, gender, and clinical data of PTB, such as fever, drug resistance, drug-induced liver damage (DILI), pulmonary infection, leukopenia, and sputum smear.
DNA extraction
Approximately 5 mL peripheral blood samples were drawn by tubes containing ethylenediaminetetraacetic acid with median cubital vein, and stored at −20 °C for DNA extraction. Then, we extracted genomic DNA from the peripheral blood leukocytes according to the standard procedures of the Flexi Gene-DNA Kit (Qiagen, Valencia, CA).
SNP selection and genotyping
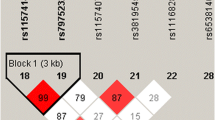
Six genes of the vitamin D metabolic pathway, including CYP24A1, CYP27A1, CYP27B1, CYP2R1, GC, DHCR7, were included in this study for analyses. We systematically searched the existing literature on the association of the polymorphisms in vitamin D metabolic pathway genes with human diseases, and looked for the SNPs associated with human disease. Then, we obtained the genotype data on these six genes of Han Chinese people in Beijing with the Ensembl Genome Browser 85 and CHBS_1000g, and used the pairwise option of the HaploView 4.0 software (Cambridge, MA, USA) for selecting the tag SNPs of each gene. Finally, we used SNP function prediction to assess the potential function of the relevant SNPs. In conclusion, we selected four SNPs (rs2248359, rs2296241, rs927650, rs6013897) of CYP24A1, two SNPs (rs17470271, rs933994) of CYP27A1, one SNPs (rs4646536) of CYP27B1, four SNPs (rs12794714, rs10741657, rs7935792, rs1562902) of CYP2R1, four SNPs (rs7041, rs3733359, rs16847024, rs4588) of GC, two SNPs (rs12785878, rs3829251) of DHCR7, respectively. All SNPs satisfied the following two conditions: minor allele frequency (MAF) ≥ 0.05 in CHB, r2 threshold > 0.8.
This study adopted improved multiple ligase detection reaction (iMLDR) genotyping assay for genotyping with the technical support of the Center for Genetic and Genomic Analysis, Genesky Biotechnologies (Inc., Shanghai). Only the individual with 100% genotyping success rate for all SNPs was included in the final analysis.
Statistical analysis
We used the Epi Data 3.1 software to enter the data, and performed the statistical analysis by SPSS 23.0 (SPSS Inc., IL, USA). Hardy-Weinberg equilibrium test of each SNP frequency among normal controls was evaluated by Chi-square. The genotype, allele frequencies differences of all SNPs between the PTB patients and normal controls were assessed by Chi-square test, and odds ratios (OR), 95% confidence intervals (CI) using the logistic regression models. We also investigated the associations between SNPs and the risk for PTB in two genetic models (dominant, recessive model), and used the SHEsis software to conduct the haplotype analysis [23]. All of the P values presented were two-sided, and P < 0.05 was considered as the threshold of statistical significance. The Bonferroni correction was used for multiple testing in SNP analysis.
Results
Our study finally included 490 PTB patients and 489 normal controls. The PTB group included 172 females and 318 males, with a mean age of 44.92±17.77, while 265 females and 224 males were enrolled in controls with a mean age of 43.43±12.95. The results showed that all the SNPs were conformed to Hardy Weinberg equilibrium in controls. The proportion of several common clinical features in patients with PTB were sputum smear-positive (26.73%), pulmonary infection (21.63%), fever (17.75%), drug resistance (15.31%), DILI (14.29%), and leukopenia (6.73%), respectively.
Association of vitamin D pathway genes SNPs with PTB
The results of allele and genotype frequencies of all the SNPs in CYP24A1, CYP27A1, CYP27B1, CYP2R1, GC, and DHCR7 genes are summarized in Table 1. We noted that the frequencies of GC rs3733359 GA genotype, rs16847024 CT genotype, rs3733359 A allele, and rs16847024 T allele were significantly decreased in PTB patients when compared with the normal controls (GA versus GG: P = 0.006; CT versus CC: P = 0.003, A versus G: P = 0.029; T versus C: P = 0.020, respectively). However, the GT genotype frequency of GC rs4588 variant in PTB patients was significantly higher than that in controls (GT versus GG: P = 0.006). Moreover, the increased risk of rs3733359, rs16847024 variants, and a decreased risk of rs4588, were found under the dominant mode (GG versus AA+GA: P = 0.006; CC versus TT+CT: P = 0.006; GG versus TT+GT: P = 0.019, respectively). GC rs7041 variant was not associated with PTB susceptibility.
As illustrated in Table 1, there were no significant differences in allele, genotype distributions of the CYP24A1 rs2248359, rs2296241, rs927650, and rs6013897 polymorphisms between the PTB patients and controls (all P values >0.05). In addition, we did not detect significant associations between CYP27A1 rs17470271, rs933994, CYP27B1 rs4646536, CYP2R1 rs12794714, rs10741657, rs7935792, rs1562902, and DHCR7 rs12785878, rs3829251 polymorphisms and the risk of PTB (all P values >0.05).
Association of vitamin D pathway gene SNPs with several clinical features among patients with PTB
We conducted a case-only analysis to explore the potential associations between the genotype, allele frequencies of CYP24A1, CYP27A1, CYP27B1, CYP2R1, GC, DHCR7 genes, and several common clinical features among PTB patients (Table S1). CYP27A1, GC, and DHCR7 genes polymorphisms were found to be significantly associated with the clinical features. For CYP27A1 gene, the rs17470271 T allele frequency was significantly lower with leukopenia (P = 0.039), and the rs933994 T allele frequency was significantly decreased with drug resistance (P = 0.047) (Table 2).
In GC gene, the CC genotype and C allele frequencies of rs7041 variant were significantly associated with the increased risk of pulmonary infection (P = 0.031, P = 0.050, respectively), and the rs3733359 A allele frequency was significantly higher with fever (P = 0.027). In addition, the TT genotype and T allele frequencies of rs16847024 were significantly increased with fever (P = 0.036, P = 0.009, respectively), and the elevated frequency of rs16847024 T allele was associated with DILI (P = 0.027). For DHCR7 gene, rs12785878 TT genotype and T allele frequencies were both significantly decreased with pulmonary infection (P = 0.047, P = 0.024, respectively). There was no significant relationship between CYP24A1, CYP27B1, CYP2R1 genes SNPs and any clinical features.
Haplotype analysis
We used the SHEsis software to detect the haplotype of CYP24A1, CYP27A1, CYP27B1, CYP2R1, GC, DHCR7 gene, and investigated the associations of these haplotypes with PTB susceptibility. Seven main haplotypes (CACT, CGCA, CGCT, CGTT, TACA, TACT, TATT) for CYP24A1, three main haplotypes (AC, AT, TT) for CYP27A1, five main haplotypes (AGAT, GAAC, GGAT, GGCC, GGCT) for CYP2R1, six main haplotypes (AACG, AATG, AGCG, AGCT, CACG, CGCG) for GC, and three main haplotypes (GA, GG, TG) for DHCR7 were detected.
The frequency distributions of these haplotypes among the PTB patients and controls are summarized in Tables 3, 4, 5, 6, and 7. The results demonstrated that the frequencies of CYP24A1 TACT and CYP2R1 GGCT haplotypes were significantly higher in the PTB patients than controls (OR = 1.25, 95% CI: 1.01-1.56, P = 0.039; OR = 1.46, 95% CI: 1.05-2.01, P = 0.023). Moreover, the frequencies of CYP2R1 GGAT and GC AATG haplotypes were found to be reduced in the PTB patients compared with controls (OR = 0.72, 95% CI: 0.54-0.95, P = 0.021; OR = 0.71, 95% CI:0.53-0.94, P = 0.016).
Discussion
At present, a wide range of research interests have been focused on the non-skeletal metabolism of vitamin D. Vitamin D could stimulate innate immunity during MTB infection, thereby controlling MTB proliferation in macrophages [24, 25]. It has also been reported to be involved in regulating the host cytotoxic T lymphocyte response and the differentiation of natural T cells into regulatory T cells, including the potential role of adaptive immunity during infection [26, 27]. Thus, some researchers speculated that vitamin D deficiency may have a causal role in increasing susceptibility to PTB. In addition, vitamin D deficiency was common in the PTB patients in Chinese population [24]. Previous studies had shown that the genetic variants of vitamin D pathway genes were correlated with the serum 25(OH)D level [28, 29]. The existing studies have concentrated only one or two vitamin D related genes in Chinese population. The association between vitamin D pathway genes polymorphisms and PTB should be further explored; this study is the first study to examine the association between 17 SNPs of vitamin D metabolic pathway genes (CYP24A1, CYP27A1, CYP27B1, CYP2R1, GC, DHCR7) and PTB susceptibility in the Chinese population.
The CYP24A1 (25-Hydroxyvitamin D-24-hydroxylase), CYP27A1 (cytochrome P450, family 27, subfamily A, polypeptide 1), CYP27B1 (25-Hydroxyvitamin D-1a-hydroxylase), CYP2R1 (vitamin D-25-hydroxylase), GC (vitamin D-binding protein, VDBP), and DHCR7 (7-dehydrocholesterol reductase) are both important genes involved in vitamin D pathway [30, 31]. In the vitamin D pathway, the DHCR7 converts 7-dehydrocholesterol to cholesterol, thus removing the cholesterol pathway from the vitamin D3 synthetic pathway. The previtamin D3 is converted to vitamin D3 in turn with a heat dependent process. As the first step, 25-hydroxylation occurred mainly in the liver, and is regulated primarily by CYP2R1 and CYP27A1. The 25(OH)D is transported to the kidneys by binding to GC, and converted through CYP27B1 to 1,25(OH)2D3 (calcitriol), which is the biologically active form. CYP24A1 could catabolize 25(OH)D, and calcitriol into biologically inactive, water-soluble metabolites for excretion in bile. These genes play important roles in vitamin D metabolism.
Sadykov et al. investigated the genetic variation of vitamin D metabolic pathway gene of the PTB patients in Kazakhstan population, and the results suggested that CYP24A1 rs6013897 variant was associated with PTB, and the interaction of CYP24A1 and VDR genetic variation might affect PTB susceptibility [22]. However, our study did not support their findings and we found no significant association of rs6013897 variant with PTB susceptibility. Furthermore, the rs2248359, rs2296241, and rs927650 polymorphisms of CYP24A1 gene were also not contributed to PTB development. In addition to race, the differences in results could be due to the different sample sizes of the two studies, and Sadykov et al. only included an insufficient sample for genetic analysis which might influenced the statistical power of this study. Haplotype analysis demonstrated that CYP24A1 TACT frequency was related to the increased risk of PTB. This indicated that CYP24A1 gene polymorphism may be involved in the pathogenesis of PTB, but its precise role remains to be further explored.
Polymorphisms in CYP2R1 gene could influence vitamin D status in the general population [28], and CYP27B1 rs4646536 T allele has been shown to be associated with vitamin D deficiency in a family-based study [32]. Moreover, there was an interaction between the CYP27A1 methylation level and serum 1,25(OH)2D level which was associated with the increased risk of PTB [11]. Thereby, we explored the role of CYP27A1 rs17470271, rs933994, CYP27B1 rs4646536, CYP2R1 rs12794714, rs10741657, rs7935792, and rs1562902 variants in the development of PTB, and no significant result was found. Similarly, a previous study showed that CYP2R1 gene polymorphism was not associated with susceptibility to TB in Pakistan [9]. Sadykov et al. reported that CYP2R1, CYP27A1, and CYP27B1 genetic variation did not affect PTB polymorphism [22]. We also investigated the possible association between these SNPs and clinical features of the PTB patients, and found that CYP27A1 rs17470271 T allele and rs933994 T allele frequencies were significantly associated with leukopenia, drug resistance in PTB patients, respectively. The emergence of drug resistance was a very noteworthy problem in the treatment of PTB, which would bring adverse effects to the treatment process. This suggests that CYP27A1 rs933994 might be used as a predictor of drug resistance in patients with PTB, and rs17470271 could also be considered as an indicator of the progression of PTB. Further research is needed to verify these hypotheses. Our results also suggested that CYP2R1 GGCT haplotype was significantly higher in patients with PTB than in normal controls, while CYP2R1 GGAT haplotype was significantly reduced. These results are very helpful to further understand the mechanism of CYP2R1 gene variation in the pathogenesis of PTB.
Vitamin D is circulated in the blood in protein in combination with VDBP, which is encoded by GC gene, and albumin. The decreased VDBP mRNA expression level along with significantly lower serum albumin level were found in active PTB patients compared to healthy controls, implying that VDBP, albumin deficiency could play a role in vitamin D deficiency states [33]. Moreover, the genetic variation of GC differs in their affinity for vitamin D metabolites in the circulation that could modulate antimycobacterial immunity [34]. Previous studies suggested that the rs7041 and rs4588 polymorphisms were not associated with PTB susceptibility [9, 22]. Our data also confirmed that rs7041 had no effect on PTB susceptibility, while rs4588 mutation was involved in the pathogenesis of PTB. In addition, our results demonstrated that rs3733359 A, rs16847024 T allele frequencies, and AATG haplotype were reduced in PTB patients compared with controls, and these SNPs might contribute to decreased susceptibility to PTB in the Chinese population. Similarly, the variations of GC rs3733359 and rs16847024 have also been proved to be the candidate susceptibility markers of gestational diabetes mellitus in Chinese women [35, 36]. In this study, the results showed that the rs7041, rs3733359, and rs16847024 were related to the occurrence of several clinical manifestations, including fever and DILI, pulmonary infection in patients with PTB. This confirmed the important role of GC gene variation in the development of PTB. Similar to the results of that study by Sadykov et al. [22], we did not find a significant association between the DHCR7 variant and the risk of PTB. It is worth noting that the significant association between DHCR7 rs12785878 and pulmonary infection, possibly suggesting a potential association between DHCR7 and PTB susceptibility.
Our study has some limitations. Firstly, the study did not exclude the potential influence of some confounding factors, such as treatment regimen, and diet. Secondly, the sample size might not be sufficient. Therefore, replication studies with larger sample size in different ethnic groups should be considered to further verify and explore the role of vitamin D metabolic pathways in PTB development.
Conclusion
Our study provided the evidence that GC rs3733359, rs16847024, and rs4588 variant might contribute to PTB susceptibility, while CYP24A1, CYP27A1, CYP27B1, CYP2R1, and DHCR7 genetic variations were not associated with the susceptibility to PTB. Moreover, several SNPs in CYP27A1, GC, and DHCR7 genes were related to multiple clinical features, including leukopenia, drug resistance, pulmonary infection, fever, and DILI, in PTB patients.
Availability of data and materials
The data generated and analyzed by this study are available from the corresponding author on reasonable request.
References
Li HM, Li Y, Zhang GY, Shi SJ, Zhang TP. Association of vitamin D receptor and CYP2R1 mRNA expression with pulmonary tuberculosis. Arch Med Sci. 2020. https://doi.org/10.5114/aoms.2020.100763
World Health Organization.2019. Global tuberculosis report 2019. https://www.who.int/tb/publications/global_report/en/.
Azad AK, Sadee W, Schlesinger LS. Innate immune gene polymorphisms in tuberculosis. Infect Immun. 2012;80(10):3343–59. https://doi.org/10.1128/IAI.00443-12.
Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20(1):581–620. https://doi.org/10.1146/annurev.immunol.20.081501.125851.
Comstock G. Tuberculosis in twins: a re-analysis of the Prophit survey. Am Rev Respir Dis. 1978;117(4):621–4. https://doi.org/10.1164/arrd.1978.117.4.621.
Fouad NA, Saeed AM, Mahedy AW. Toll like receptor-4 gene polymorphism and susceptibility to pulmonary tuberculosis. Egypt J Immunol. 2019;26(2):1–10.
Wang W, Deng G, Zhang G, Yu Z, Yang F, Chen J, et al. Genetic polymorphism rs8193036 of IL17A is associated with increased susceptibility to pulmonary tuberculosis in Chinese Han population. Cytokine. 2020;127:154956.
Liu Y, Zhao E, Zhu L, Zhang D, Wang Z. 3'UTR polymorphisms in NRAMP1 are associated with the susceptibility to pulmonary tuberculosis: a MOOSE-compliant meta-analysis. Medicine (Baltimore). 2019;98:e15955.
Junaid K, Rehman A, Jolliffe DA, Saeed T, Wood K, Martineau AR. Vitamin D deficiency associates with susceptibility to tuberculosis in Pakistan, but polymorphisms in VDR, DBP and CYP2R1 do not. BMC Pulm Med. 2016;16(1):73. https://doi.org/10.1186/s12890-016-0240-2.
Tessema B, Moges F, Habte D, Hiruy N, Yismaw S, Melkieneh K, et al. Vitamin D deficiency among smear positive pulmonary tuberculosis patients and their tuberculosis negative household contacts in Northwest Ethiopia: a case-control study. Ann Clin Microbiol Antimicrob. 2017;16(1):36. https://doi.org/10.1186/s12941-017-0211-3.
Wang M, Kong W, He B, Li Z, Song H, Shi P, et al. Vitamin D and the promoter methylation of its metabolic pathway genes in association with the risk and prognosis of tuberculosis. Clin Epigenetics. 2018;10:118.
Keflie TS, Nolle N, Lambert C, Nohr D, Biesalski HK. Vitamin D deficiencies among tuberculosis patients in Africa: a systematic review. Nutrition. 2015;31(10):1204–12. https://doi.org/10.1016/j.nut.2015.05.003.
Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37(1):113–9. https://doi.org/10.1093/ije/dym247.
Bellamy R, Ruwende C, Corrah T, McAdam K, Thursz M, Whittle H, et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179(3):721–4. https://doi.org/10.1086/314614.
Selvaraj P, Harishankar M, Afsal K, Vitamin D. Immuno-modulation and tuberculosis treatment. Can J Physiol Pharmacol. 2015;93(5):377–84. https://doi.org/10.1139/cjpp-2014-0386.
Bivona G, Agnello L, Bellia C, Iacolino G, Scazzone C, Lo Sasso B, et al. Non-skeletal activities of vitamin D: from physiology to brain pathology. Medicina (Kaunas). 2019;55(7).
Bivona G, Agnello L, Ciaccio M. The immunological implication of the new vitamin D metabolism. Cent Eur J Immunol. 2018;43(3):331–4. https://doi.org/10.5114/ceji.2018.80053.
Silva-Ramírez B, Saenz-Saenz CA. Bracho-Vela LA Association between vitamin D receptor gene polymorphisms and pulmonary tuberculosis in a Mexican population. Indian J Tuberc. 2019;66(1):70–5. https://doi.org/10.1016/j.ijtb.2018.04.005.
Wang Y, Li HJ. A meta-analysis on associations between vitamin D receptor genetic variants and tuberculosis. Microb Pathog. 2019;130:59–64. https://doi.org/10.1016/j.micpath.2019.02.027.
Zhou TB, Jiang ZP, Li AH, Ju L. Vitamin D receptor ApaI (rs7975232), BsmI (rs1544410), Fok1 (rs2228570), and TaqI (rs731236) gene polymorphisms and susceptibility to pulmonary tuberculosis in an Iranian population: a systematic review and meta-analysis. J Recept Signal Transduct Res. 2015;35(2):107–14. https://doi.org/10.3109/10799893.2014.936459.
Hu QY, Chen ZS, Liang GNA, Mo FP, Zhang HX, Xu SL, et al. Vitamin D receptor gene associations with pulmonary tuberculosis in a Tibetan Chinese population. BMC Infect Dis. 2019;16.
Sadykov M, Azizan A, Kozhamkulov U, Akilzhanova A, Yerezhepov D, Salfinger M, et al. Association of genetic variations in the vitamin D pathway with susceptibility to tuberculosis in Kazakhstan. Mol Biol Rep. 2020;47(3):1659–66. https://doi.org/10.1007/s11033-020-05255-3.
Li Z, Zhang Z, He Z, Tang W, Li T, Zeng Z, et al. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis (http://analysis.bio-x.cn). Cell Res. 2009;19(4):519–23. https://doi.org/10.1038/cr.2009.33.
van der Does AM, Bergman P, Agerberth B, Lindbom L. Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. J Leukoc Biol. 2012;92(4):735–42. https://doi.org/10.1189/jlb.0412178.
Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–12. https://doi.org/10.4049/jimmunol.173.5.2909.
Sarkar S, Hewison M, Studzinski GP, Li YC, Kalia V. Role of vitamin D in cytotoxic T lymphocyte immunity to pathogens and cancer. Crit Rev Clin Lab Sci. 2016;53(2):132–45. https://doi.org/10.3109/10408363.2015.1094443.
Wang Q, Ma A, Gao T, Liu Y, Ren L, Han L, et al. Poor vitamin D status in active pulmonary tuberculosis patients and its correlation with Leptin and TNF-α [J]. J Nutr Sci Vitaminol. 2019;65(5):390–8. https://doi.org/10.3177/jnsv.65.390.
Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–8. https://doi.org/10.1016/S0140-6736(10)60588-0.
Thongthai P, Chailurkit LO, Chanprasertyothin S, Nimitphong H, Sritara P, Aekplakorn W, et al. Vitamin D binding protein gene polymorphism as a risk factor for vitamin D deficiency in Thais. Endocr Pract. 2015;21(3):221–5. https://doi.org/10.4158/EP14266.OR.
Bosse Y, Lemire M, Poon AH, Daley D, He JQ, Sandford A, et al. Asthma and genes encoding components of the vitamin D pathway. Respir Res. 2009;10(1):98. https://doi.org/10.1186/1465-9921-10-98.
Vimaleswaran KS, Cavadino A, Berry DJ. Genetic association analysis of vitamin D pathway with obesity traits. Int J Obes. 2013;37(10):1399–406. https://doi.org/10.1038/ijo.2013.6.
Yu SC, Li X, Wang Y, Mao ZX, Xie YC, Zhang L, et al. Family-based association between allele T of rs4646536 in CYP27B1 and vitamin D deficiency. J Clin Lab Anal. 2019;33(6):e22898.
Panda S, Tiwari A, Luthra K, Sharma SK, Singh A. Status of vitamin D and the associated host factors in pulmonary tuberculosis patients and their household contacts: a cross sectional study. J Steroid Biochem Mol Biol. 2019;193:105419. https://doi.org/10.1016/j.jsbmb.2019.105419.
Martineau AR, Leandro ACCS, Anderson ST, Newton SM, Wilkinson KA, Nicol MP, et al. Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J. 2010;35(5):1106–12.
Wang Y, Wang O, Li W, Ma L, Ping F, Chen L, et al. Variants in vitamin D binding protein gene are associated with gestational diabetes mellitus. Medicine (Baltimore). 2015;94(40):e1693. https://doi.org/10.1097/MD.0000000000001693.
Shi A, Wen J, Liu G, Liu H, Fu Z, Zhou J, et al. Genetic variants in vitamin D signaling pathways and risk of gestational diabetes mellitus. Oncotarget. 2016;7(42):67788–95. https://doi.org/10.18632/oncotarget.11984.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82003515) and Anhui Provincial Natural Science Foundation (1908085QH368).
Author information
Authors and Affiliations
Contributions
T-PZ and H-ML designed the study. H-ML conducted the experiment. S-SC performed the statistical analyses. G-YZ and S-JS participated in sample collection. T-PZ drafted the manuscript. T-PZ and LW contributed to the manuscript revision. All authors approved the final submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Anhui Chest Hospital (K2020-005), and the informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors confirm that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The associations between vitamin D pathway gene polymorphisms and clinical features of PTB patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, TP., Chen, SS., Zhang, GY. et al. Association of vitamin D pathway genes polymorphisms with pulmonary tuberculosis susceptibility in a Chinese population. Genes Nutr 16, 6 (2021). https://doi.org/10.1186/s12263-021-00687-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12263-021-00687-3