Abstract
Background
Cardiopulmonary collapse is a catastrophic event in cesarean section, which leads to adverse outcomes for both the mother and the fetus. Pulmonary embolism is one of the rare etiologies of this entity. We herein reported the successful management of acute embolism pulmonary associated with cesarean delivery on a healthy pregnant woman at our tertiary referral hospital.
Case presentation
A full-term pregnant woman hospitalized for planned cesarean delivery due to placenta previa without cardiorespiratory diseases. She was scheduled uneventfully for a planned cesarean section. After placental delivery, the patient spontaneously fell into cardiopulmonary collapse and her vital signs deteriorated rapidly. The obstetricians promptly completed the cesarean section and performed all procedures to prevent the PPH and preserve the uterus. At the same time, the anesthesiologists continued to carry out advanced heart-lung resuscitation in order to control her vital signs. After surgery, the multidisciplinary team assessed the patient and found a thrombus in her pulmonary circulation. Therefore, the patient was managed with therapeutic anticoagulation. The patient recovered in good clinical condition and was discharged after 2 weeks without any complications.
Conclusions
The diagnosis of acute pulmonary embolism is extremely difficult due to uncommon occurrence, sudden onset, and non-specific presentation. Awareness of this life-threatening pathology during cesarean delivery should be raised. Interdisciplinary assessment must be essentially established in this life-threatening condition. After the whole conventional management, uterine conservation may be acceptable where applicable. Further data is required to encourage this finding.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Both cardiopulmonary collapse and sudden cardiorespiratory arrest are unusual during delivery. They are even more life-threatening in low-resource settings [1, 2]. Proper management of the critically ill patient in cardiopulmonary collapse requires rapid identification of its etiology. Etiologies include obstetric and non-obstetric events [3, 4]. In the obstetric field, cardiopulmonary collapse may be caused by pulmonary embolism (PE) or amniotic fluid embolism (AFE) during labor. Some risk factors include abdominal surgery, obesity, hypertension, severe preeclampsia, chronic medical diseases, and prolonged immobilization [5]. Hypercoagulability in pregnancy has been estimated to increase the risk of venous thromboembolism by about fivefold. However, it is infrequent among healthy women [6, 7]. The incidence of venous thromboembolism (VTE) was 0.4 per 1000 pregnancies, of which 83.3% were deep vein thrombosis and 16.7% were pulmonary embolism in Chinese pregnant women [8]. According to the report of Morikawa and others, the incidence of VTE after cesarean delivery (0.0074%) was significantly higher than that after vaginal delivery (0.0012%) [9, 10]. Due to its rarity, awareness of this pathology is low among reproductive-age and pregnant women, particularly, in low-middle-income countries (LMICs) [11]. APE is often rapidly fatal [12, 13]. A delayed recognition could lead to maternal death, but a misdiagnosis results in overtreatment and neglect of other pathologies [14]. According to a report of Elgendy, the rates of in-hospital mortality were almost 200-fold higher among those who had APE (29.3 vs 0.13, per 1000 pregnancy-related, P < 0.001) and the rates of in-hospital mortality have not improved (2.6% in 2007 vs 2.5% in 2015, Ptrends = 0.74) [15]. If APE occurs before delivery, both maternal–fetal outcomes are dramatically poor [16]. Clinically, the prognosis of PE is better than AFE. PE can be treated with timely intervention but requires early recognition. PE contributes highly to peripartum maternal death [17, 18]. The suspicion of PE can be confirmed by imaging modalities, Wells score, D-dimer, and histopathological endpoints [19, 20].
PE can occur before delivery, during delivery, and in the postpartum period [16, 21]. However, the rarest is PE during cesarean section (C-section). Optimal management requires timely recognition, and treatment requires a multidisciplinary team which involves the coordinated action of the cardiologist, obstetrician, anesthesiologist, and surgeon. An implemented treatment includes thrombolysis and anticoagulation [22]. Early surgical embolectomy and bridged with extracorporeal membrane oxygenation may be required in some severe cases [23,24,25]. Cesarean hysterectomy is often undertaken due to the massive active bleeding after failed conservative treatment, coagulation disturbances, and a high risk of postpartum hemorrhage (PPH) that treatment of PE requires [26]. Nevertheless, hysterectomy is not optimal for a young woman who desires to preserve fertility. Meanwhile, the paucity of strong evidence in the medical literature makes it difficult to assess the safety of conservative treatment in this emergency.
Hereby, we present an uncommon case of acute embolism pulmonary during cesarean section in an otherwise healthy woman and thereby contribute to the literature on the possibility of uterine preservation where applicable.
Case presentation
A 36-year-old woman, Gravida 3 Para 1, spontaneous abortion 2, was admitted to Tu Du Hospital due to spontaneous labor with vaginal bleeding sign. The patient’s medical history was otherwise unremarkable. Her obstetric history recorded a previous cesarean section for breech presentation and two miscarriages. At hospitalization, the patient had normal vital signs. She was at term after an uncomplicated gestation except for placenta previa Grade I. She had a known abnormal placentation site at 28 weeks of gestational age.
Therefore, an emergency C-section was scheduled. Before surgery, the patient was normotensive with a pulse of 82 beats per minute (bpm) and respiratory rate of 20 times per min. Her pre-pregnancy body mass index (BMI) was 21.6 kg/m2. In this pregnancy, her BMI was 25.6 kg/m2 (maternal weight: 54 kg, maternal height: 145 cm). Her legs were noted without gross varicose veins. Complete blood count was normal; coagulation parameters included prothrombin time (PT) of 98%, international normalized ration (INR) of 1.01, temps de quick (TQ) of 13.4 s, activated partial thromboplastin time (APTT) of 28.8 s, and plasma fibrinogen of 480 mg/dL. Electrocardiogram (ECG) showed sinus tachycardia of 116 bpm. The chest radiograph was normal. Obstetric examination revealed infrequent episodes of mild uterine contraction, a closed cervix, and intact membranes. Ultrasound showed normal fetal development in transverse lie, with an estimated weight of 3100 g with normal Doppler blood flow. The cardiotocography (CTG) proceeded as Group I by the American College of Obstetricians and Gynecologists criteria (ACOG 2009). The airway was Mallampati grade II.
At the beginning of the cesarean delivery, her blood pressure was at 130/80 mmHg, pulse rate was 100 bpm, and peripheral transcutaneous oxygen saturation (SpO2) was 100%. The patient underwent general anesthesia with tracheal intubation. After 10 min of surgery, a healthy male newborn weighing 3100 g was delivered with reported Apgar scores of 7 and 8 at 1 and 5 min, respectively, and a normal placenta was gently extracted. Transparent amniotic fluid was approximately 500 ml. However, the patient’s cardiopulmonary system collapsed immediately after placental extraction. At this time, her vital signs deteriorated with blood pressure (BP) at 90/60 mmHg, tachycardia at 125 beats/min, and SpO2 suddenly dropping to 86%. Shock index (SI) of 1.4 (0.5–0.7). Therefore, the initial differential diagnosis was amniotic embolism versus acute pulmonary embolism (Table 1). A “Red Code” was called by anesthesia while the obstetricians continued to manage the surgery. A multidisciplinary team managed the resuscitation. She responded to a total of 30 mg doses of ephedrine.
During surgery, the obstetrician focused on hemostasis carefully. Bilateral artery ligation, placental bed sutures, and B-Lynch compression sutures were all performed due to the high risk of PPH. Uterotonic drugs including oxytocin, methyl ergonovine, carboprost, and tranexamic acid were administered. The uterus was contracted, so the team decided to preserve the uterus. The uterus and abdomen were quickly and carefully closed to minimize the surgical time. An abdominal drain was placed. Total estimated blood loss was measured at 500 ml and surgical time duration was 100 min. Urine output was measured at 100 ml during surgery.
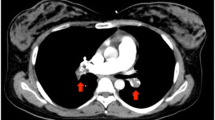
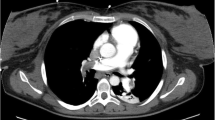
Meanwhile, urgent transthoracic echocardiography showed a sizable thrombus in the pulmonary vasculature, in the subclavian vein, in the right jugular vein, and in the right ventricular cavity (Fig. 1 and Supplementary videos 1, 2, 3, and 4, respectively). Thus, a diagnosis of pulmonary embolism was established. Ultrasonic assessment demonstrated no abnormalities in the lower limb veins.
After surgery, the patient was transferred immediately to the intensive care unit (ICU) where she was cared for by a multidisciplinary team consisting of obstetrician, anesthetist, cardiologist, hematologist, and sonographer. Ventilation was maintained by a positive pressure-supported respirator. She was sedated, paralyzed, and maintained with a narcotic, an anticonvulsant drug, and a muscle relaxant drug (Fentanyl 100 mg 02 ampules combined with Midazolam 5 mg 05 ampules intravenous infusion at 6 ml/h, with Rocuronium 10 mg/ml × 5 ampules in 45 ml sodium chloride 0.9% for 50 ml infused via an intravenous electric pump at 10 ml/h). Additional analgesia included subcutaneous morphine sulfate 10 mg and diclofenac 100-mg rectal suppository.
D-dimer levels were markedly increased to 46,700 ng/ml. The coagulation profile showed a weak to moderate blood clot contraction with PT = 62%, INR = 1.29, TQ = 13.8 s, TCK = 34 s, and fibrinogen = 136 mg/dl. Hemoglobin level fell to 7.6 g/dl. The patient was transfused with 2 units of AB-positive packed red blood cells (350 ml/unit) and was given 4 packs of fresh frozen plasma (200 ml/unit), as well as all 10 packs of cryoprecipitate (50 ml/unit). The patient received a therapeutic dose of one ampule of low-molecular-weight heparin (25,000 IU/5 ml/ampule). The abdomen drained 200 ml of abdominal cavity fluid without active bleeding. An intravenous broad-spectrum antibiotic (tarzocin 4.5 g/ampule every 6 h) was given to prevent the secondary infection.
After counseling with an interdisciplinary team, the patient was transferred to Cho Ray Hospital, a tertiary general hospital for further management after 16 h of obstetric and hemodynamic stabilization. At the tertiary center, chest X-ray was normal, and cardiac ultrasound revealed mild right ventricular dilation and normal left systolic function. Unfortunately, the computed tomographic pulmonary angiography (CTPA) confirmed nearly total occlusion of segmental arterial branches from the upper lobes of both lungs, the left and the right lower lobar branches. The descending branch of the left pulmonary artery was partially occluded; the lateral basal and the posterior basal arterial branches of the left lower lobe were completely occluded. Both lungs had small pleural effusions and passive atelectasis of inferior areas. Abdominal ultrasound showed free air, infiltration, and a small amount of fluid in the lower right abdomen. The hepatic and renal function tests as well as the electrolyte profile were within normal limits. However, high sensitivity Troponin 1 and NT-proBNP were elevated at 637.5 pg/mL and 96.75 pmol/L, respectively. The complete blood count test showed moderate anemia with Hb of 79 g/L (7.9 g/dL), an increased white blood cell count (WBC) of 17.69 G/L, and a low platelet count of 119 G/L.
At the tertiary hospital, the patient received 2 additional units of blood and was intensively treated with anti-coagulant therapy. Specifically, heparin 10,000 IU was first administered with 50 ml sodium chloride 0.9% solution, giving a bolus of 15 ml (3000 IU) and maintenance of 4–6 ml per hour (18 IU/kg) monitored by activated clotting time (ACT) levels (147–172 s). On the second day, the patient was extubated; oxygenation was supported with a nasal cannula. Heparin was stopped. Lovenox 60 mg/0.6 ml was subcutaneously injected twice a day at 12-h intervals. Additionally, she received pantoprazole for gastroesophageal reflux disease (GERD) during her admission to the intensive care unit. She was discharged after 2 weeks in good health, with normal consciousness and without neurological injuries. The patient and her family felt grateful to the team for saving her life.
Discussion
Cardiopulmonary complications of C-sections are a leading cause of maternal morbidity and mortality, particularly in low-middle-income countries or in low-resource settings [2]. Almost all cases of thromboembolism (TE) are diagnosed during the postpartum period. An APE that occurs immediately during C-section seems rarely to be reported. This pathology is mainly documented through a case by case in the literature [7, 27, 28]. According to the retrospective study of Lai et al., out of 1377 pregnant women who underwent C-section, only 7 cases developed venous TE and 86% of cases were within 7 days of postpartum. This study revealed that hypertension and the presence of varicose veins were associated with TE following C-section [29]. In our case, several risk factors contributed to this woman’s cardiorespiratory collapse. First, an advanced maternal age > 35 years old, previous cesarean delivery, and C-section due to placenta previa in this pregnancy (Table 2). After the coronavirus disease 2019 (COVID-19) pandemic, the incidence of VTE seems likely to increase [30,31,32]. Particularly, the PE cases associated with the placental implantation site have been recorded recently at our center. Placenta previa resulting in a massive PPH may be a high risk for VTE [33, 34]. However, none of the similar reports has been documented in the literature. Therefore, the underlying mechanism of this occurrence due to hidden blood clots from the deep veins before surgery or from placenta previa after placental delivery remains controversial.
A timely diagnosis of APE remains totally difficult since it is overlapped with other complications and is camouflaged by the physiological changes in pregnancy [16]. In the case of a parturient with signs of sudden cardiopulmonary arrest during C-section, the initial differential diagnosis includes anaphylaxis, pulmonary embolism (PE), and acute coagulopathy and even cerebral hemorrhage due to amniotic fluid embolism, or cerebral hemorrhage due to severe preeclampsia. However, this patient did not suffer hypertension during pregnancy. The patient showed no immediate signs of allergic reaction to the general anesthesia and the collapse did not occur immediately.
Clinically, the catastrophic manifestation of PE and AFE is similar. In the current case, since the patient was under general anesthesia, the initial clinical symptoms such as dyspnea, shortness of breath, and chest pain were absent. Diagnosis criteria in AFE have been studied [47]. According to the Society of Maternal Fetal Medicine (SMFM) and the Amniotic Fluid Embolism Foundation, the four proposed diagnostic criteria for AFE include (1) sudden cardiac arrest or both respiratory and hemodynamic collapse, (2) disseminated intravascular coagulopathy (DIC), (3) absence of fever, and (4) clinical onset during labor or within 30 min of delivery [12, 48]. Well’s diagnostic criteria for PE were met in our patient [20]. An increased D-dimer is a valuable predictive marker for PE (Table 1). The D-dimer cut-off value of 800 ng/mL ensures high sensitivity and increases specificity compared to the conventional threshold of 500 ng/mL. Considering this higher threshold can reduce the number of unnecessary CT scans and subsequently unnecessary radiation exposure in women after Cesarean delivery [49, 50]. Nevertheless, the value of D-dimer is still limited since the concentration depends on the gestational age [14]. Recently, the value of the Fibrin monomer test has also been a concern since its concentration is nearly unchanged during pregnancy [51]. However, our patient was assessed with a low risk of VTE during the antenatal period following The Royal College of Obstetricians and Gynaecologists (RCOG Green-top Guideline, no. 37a. Reducing the risk of thrombosis and embolism during pregnancy and the puerperium. London: RCOG, 2015); thus, the screening modalities were not indicated [34]. According to local guidelines, no routine thromboprophylaxis was given during the antenatal care. Moreover, the patient was in emergent condition with hemorrhage originating from placenta previa. Thus, the surgery was a priority.
But more than 90% of pregnant women with suspected PE do not have PE [14]. The gold standard for diagnosis of PE is found at autopsy. Fortunately, the patient lived, so the only evidence for PE was the ultrasonic findings of the blood clot in the heart and pulmonary artery. Ultrasound can be performed at the bedside more rapidly than a CT scan and so is a more useful diagnostic tool in a critical situation [19]. CT angiopathy can be indicated to confirm the diagnosis of APE when the patient is in stable condition [7, 24, 52].
Ultrasound can also help differentiate between PE and AFE. Histopathologically, AFE material can include amniotic fluid, as well as fetal cells, hair, or other debris entering the mother’s bloodstream through the placental bed. It is usually rapidly progressive and has a very high mortality [53]. These materials are sonographically different in appearance from a clot. In our patient, no strange structure was on ultrasound. Thus, cardiopulmonary collapse due to PE was the most likely diagnosis in this pregnant woman.
The neonatal outcome should be mentioned. In this case, PE occurred after fetal extraction. The infant was unaffected. However, when PE occurs prior to cord clamping, the wellbeing of both mother and child is at risk. Emergency C-section may be necessary to save the newborn life. Additionally prompt delivery improves maternal outcome by reducing maternal vena cava compression due to the gravida uterus, thus increasing maternal cardiac output, and may improve the hypercoagulability of pregnancy [21, 46, 53].
The modern management of PE includes anti-coagulation, thrombolysis, and surgical pulmonary embolectomy. The choice of management should be based on the patient’s status and the size of the thrombus clot [26, 38]. However, no practical guidelines have been established for this difficult entity [46]. Thrombolytic therapy could be used if the PE occurs before labor [18]. Remarkably, thrombolysis and administration of tissue plasminogen activator may be contraindicated at the onset of massive hemorrhage during surgery [23, 24]. Postpartum thrombolytic therapy with recombinant tissue plasminogen activator (rt-PA) needs to be strictly monitored because the treatment may lead to massive bleeding [25, 54]. In the present case, anticoagulant treatment using intravenous heparin and low molecular weight heparin subcutaneous injection were indicated during obstetric stabilization without excessive vaginal bleeding.
In general, it is a risk to conserve the uterus in a pregnant woman with PE because of the high risk of PPH and the associated coagulopathies. Previously, almost all reports have recommended emergent cesarean hysterectomy in the management of pulmonary embolism during cesarean delivery. Intra-uterine tamponade using a Bakri® postpartum balloon may be performed for temporary hemostasis [23]. In this case, the patient was administered 1 g of tranexamic acid as a routine drug in the PPH during C-section. The team did not assess the pulmonary embolism at the initial moment. However, the risks and benefits of this drug administration are still controversial due to the risk of thrombosis [55,56,57]. Karakosta et al. have recently reported a case of uterine conservation after acute life-threatening PE during C-Section which required postpartum rescue hysterectomy due to massive bleeding [26]. Initial uterine conservation carries the risk of subsequent emergency hysterectomy. Immediate resuscitation and coagulopathy monitoring is essential. Life-saving treatment must always be a priority over future fertility. However, when there is a multidisciplinary team immediately available and when immediate surgery could be undertaken if necessary, uterine conservation with preservation of future fertility can be considered. In addition, aside from uterotonic drugs, the time duration between a cesarean hysterectomy and all hemostatic procedures in uterine conservative surgery is also a main concern since it relates directly to maternal outcomes. In our case, the total surgical time duration from the onset of APE to abdominal closure was 110 min. Due to a lack of evidence, the team could not compare this surgical time to any documents in the literature. Further studies are required to clarify this entity. Nevertheless, regarding the surgical methods, the hemostatic procedures relating to this surgery were mentioned in a recent article. In this article, the total time duration of the uterine conservative surgery was shorter than that of a cesarean hysterectomy [58].
Particularly, postpartum care needs to be strictly monitored in APE cases due to the high risk of PPH and infectious post-surgery. Song et al. have reported a case of obstructive uropathy caused by a 20-cm-sized hematoma anterior to the bladder in Retzius space following postpartum pulmonary embolism [59]. Recently, elevated plasma lipocalin-2 levels have been a promising biomarker in predicting long-term major adverse events among normotensive patients with APE for risk stratification in the intermediate-risk group [60]. Most importantly, multidisciplinary management with interhospital transfer and advanced armamentarium is necessarily required to control the coagulopathy profile, prevent multi-organ dysfunction, and reduce significant mortality [23, 61]. Maintenance therapy using LMWH or warfarin should be continued for at least 6 weeks postnatal and for a minimum of 3 months in total as these have proven safe in breastfeeding women. The subsequent pregnancy needs to be treated with VTE prophylaxis [62].
Conclusions
The diagnosis of APE remains a challenge due to its rarity, sudden onset, and non-specific symptoms. A high index of suspicion for acute pulmonary embolism during cesarean delivery is potentially necessary, even in any parturient woman. Immediate interdisciplinary resuscitation can minimize maternal death. Although cesarian hysterectomy has been the standard treatment, when the right resources are present, uterine conservation may be considered. Further data is required to determine under which conditions cesarean hysterectomy or uterine conservative management is better.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AFE:
-
Amniotic fluid embolism
- APE:
-
Acute pulmonary embolism
- CS or C-section:
-
Cesarean section
- PE:
-
Pulmonary embolism
- PPH:
-
Postpartum hemorrhage
- GA:
-
Gestational age
- US:
-
Ultrasound
- VTE:
-
Venous thromboembolism
References
Sharabi AF, Singh A. ardiopulmonary arrest in adults. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023; 2023.
Nivatpumin P, Lertbunnaphong T, Dittharuk D. A ten-year retrospective review of maternal cardiac arrest: Incidence, characteristics, causes, and outcomes in a tertiary-care hospital in a developing country. Taiwan J Obstet Gynecol. 2021;60(6):999–1004.
Al Dandan O, Hassan A, AbuAlola H, Alzaki A, Alwaheed A, Alalwan M, et al. Clinical and imaging profiles of pulmonary embolism: a single-institution experience. Int J Emerg Med. 2020;13(1):47.
Ebhohon E, Miller D. Moringa Oleifera leaf extract induced pulmonary embolism-a case report. Int J Emerg Med. 2022;15(1):16.
Doherty S. Pulmonary embolism an update. Aust Fam Physician. 2017;46(11):816–20.
Taimur SDM, Haq MM, Khan SR, Kabir CMS, Rahman H, Karim MR, et al. A young lady with acute pulmonary embolism after caesarean section - a case report. BIRDEM Med J. 2013;3(2):116–20.
Krawczyk P, Huras H, Jaworowski A, Tyszecki P, Kołak M. Cesarean section complicated with presumed massive pulmonary embolism and cardiac arrest treated with rescue thrombolytic therapy-two case reports. Ann Palliat Med. 2023;12(1):219–26. https://doi.org/10.21037/apm-22-435.
Huang D, Wong E, Zuo ML, Chan PH, Yue WS, Hu HX, et al. Risk of venous thromboembolism in Chinese pregnant women: Hong Kong venous thromboembolism study. Blood Res. 2019;54(3):175–80.
Morikawa M, Adachi T, Itakura A, Nii M, Nakabayashi Y, Kobayashi T. A retrospective cohort study using a national surveillance questionnaire to investigate the characteristics of maternal venous thromboembolism in Japan in 2018. BMC Pregnancy Childbirth. 2021;21(1):514.
Evangelista MS, Slompo K, Timi JRR. Venous thromboembolism and route of delivery - review of the literature. Rev Bras Ginecol Obstet. 2018;40(3):156–62.
Wani JI, Nadeem M. Awareness regarding venous thromboembolism and pulmonary embolism after pregnancy and cesarean section in female population in the Aseer Region, Saudi Arabia. Cureus. 2023;15(12):e51272.
Clark SL, Romero R, Dildy GA, Callaghan WM, Smiley RM, Bracey AW, et al. Proposed diagnostic criteria for the case definition of amniotic fluid embolism in research studies. Am J Obstet Gynecol. 2016;215(4):408–12.
Fitzpatrick KE, Tuffnell D, Kurinczuk JJ, Knight M. Incidence, risk factors, management and outcomes of amniotic-fluid embolism: a population-based cohort and nested case–control study. BJOG. 2016;123(1):100–9.
Robert-Ebadi H, Moumneh T, Le Gal G, Righini M. Diagnosis of pulmonary embolism during pregnancy. Diagnostics (Basel, Switzerland). 2022;12(8):1875.
Elgendy IY, Gad MM, Mansoor H, Mahmoud AN, Elbadawi A, Saad A, et al. Acute pulmonary embolism during pregnancy and puerperium: national trends and in-hospital outcomes. Mayo Clin Proc. 2021;96(8):2102–13.
dos Santos LF, Andrade C, Rodrigues B, Moreira D, Delgado A, Manso P, et al. Pregnancy and acute pulmonary embolism: a case report. Re Port Cardiol. 2012;31(5):389–94.
Heyl PS, Sappenfield WM, Burch D, Hernandez LE, Kavanaugh VM, Hill WC. Pregnancy-related deaths due to pulmonary embolism: findings from two state-based mortality reviews. Matern Child Health J. 2013;17(7):1230–5.
Cueto-Robledo G, Cervantes-Naranjo FD, Gonzalez-Hermosillo LM, Roldan-Valadez E, Graniel-Palafox LE, Castro-Escalante KY, et al. Pulmonary embolism during pregnancy: an updated review with case series description. Curr Probl Cardiol. 2023;48(7):101683.
Squizzato A, Galli L, Gerdes VEA. Point-of-care ultrasound in the diagnosis of pulmonary embolism. Crit Ultrasound J. 2015;7(1):7.
van Es N, Kraaijpoel N, Klok FA, Huisman MV, Den Exter PL, Mos ICM, et al. The original and simplified Wells rules and age-adjusted D-dimer testing to rule out pulmonary embolism: an individual patient data meta-analysis. J Thromb Haemost. 2017;15(4):678–84.
Sitras V, Raatiniemi L, Larsby K, Klingenberg C. Cardiopulmonary collapse during labour. Anesthesiol Res Pract. 2010;2010:707619.
Fasullo S, Maringhini G, Terrazzino G, Ganci F, Paterna S, Di Pasquale P. Thrombolysis for massive pulmonary embolism in pregnancy: a case report. Int J Emerg Med. 2011;4:69.
Colombier S, Niclauss L. Successful surgical pulmonary embolectomy for massive perinatal embolism after emergency cesarean section. Ann Vasc Surg. 2015;29(7):1452.e1–4.
Park JH, Hong SC, Yun HY, Jeon YG, Kim S, Song SW. Massive pulmonary embolism after caesarean section managed with surgical thrombectomy bridged with extracorporeal membrane oxygenation: a case report. Int J Surg Case Rep. 2023;107:108371.
Ho YK, Wang CP, Wu YL, Lee TH, Ying TH, Lee MS. Pulmonary embolism after cesarean section and successful treatment with early application of extracorporeal membrane oxygenation system and anticoagulant agents. Taiwan J Obstet Gynecol. 2014;53(2):273–5.
Agathi K, Theocharis E, Stefanos F, Ioanna S, Effrosyni S, Alexandros D, et al. Systemic thrombolysis for treatment of acute life-threatening pulmonary embolism during cesarean section followed by post-partum rescue hysterectomy: a case report and review of the literature. In Vivo. 2023;37(1):498.
Pandey S, Sharma J, Manandhar BL, Adhikari A. Acute pulmonary embolism after cesarean section. J Nepal Health Res Counc. 2015;13(31):241–4.
Song, Bin; Sun, Yue; Liu, Dandan; Li, Guanggang∗. Acute pulmonary embolism immediately after cesarean section despite dilatation of the left ventricle: a case report and literature review. Emerg Crit Care Med. 2023;3(3):130–5. https://doi.org/10.1097/EC9.0000000000000073.
Lai J, Venu I, Malinowski AK, Gandhi S, McLeod A, Nisenbaum R, et al. Thromboembolism following cesarean section: a retrospective study. Hematology (Amsterdam, Netherlands). 2018;23(6):351–6.
Angelini DE, Kaatz S, Rosovsky RP, Zon RL, Pillai S, Robertson WE, et al. COVID-19 and venous thromboembolism: a narrative review. Res Pract Thromb Haemost. 2022;6(2):e12666.
Cui LY, Cheng WW, Mou ZW, Xiao D, Li YY, Li YJ, et al. Risk factors for pulmonary embolism in patients with COVID-19: a systemic review and meta-analysis. Int J Infect Dis. 2021;111:154–63.
Perfilyeva YV, Maukayeva SB, Smail YM, Dmitrovskiy AM, Ostapchuk YO, Zhigailov AV, et al. Lethal pulmonary embolism in a pregnant woman with severe acute respiratory syndrome coronavirus-2 receiving prophylactic anticoagulation: a case report. J Med Case Reports. 2023;17(1):455.
Park HS, Cho HS. Management of massive hemorrhage in pregnant women with placenta previa. Anesth Pain Med. 2020;15(4):409–16.
Lamont MC, McDermott C, Thomson AJ, Greer IA. United Kingdom recommendations for obstetric venous thromboembolism prophylaxis: Evidence and rationale. Semin Perinatol. 2019;43(4):222–8.
van Liempt SW, Stoecklein K, Tjiong MY, Schwarte LA, de Groot CJ, Teunissen PW. Essentials in cardiac arrest during cesarean section. Clin Pract. 2015;5(1):668.
Wang QM, Liu HL, Dang Q. Acute trophoblastic pulmonary embolism during conservative treatment of placenta accreta: case report and review of literature. Eur J Med Res. 2015;20:91.
Yufune S, Tanaka M, Akai R, Satoh Y, Furuya K, Terui K, et al. Successful resuscitation of amniotic fluid embolism applying a new classification and management strategy. JA Clin Rep. 2015;1(1):1.
Ahn KH, Hong SC. Embolectomy for massive pulmonary embolism after cesarean delivery. Can Med Assoc J. 2016;188(4):E73.
Umazume T, Hayasaka S, Kato F, Ishikawa S, Morikawa M, Minakami H. Sudden maternal hypoxemia during elective cesarean section in a woman with placenta previa. Clin Case Rep. 2017;5(10):1668–71.
Oda Y, Fujita M, Motohisa C, Nakata S, Shimada M, Komatsu R. Pulmonary embolism caused by ovarian vein thrombosis during cesarean section: a case report. JA Clin Rep. 2018;4(1):3.
Tong A, Zhao F, Liu P, Zhao X, Qi X. Management of postpartum pulmonary embolism combined with retained placenta accreta: A case report. Medicine (Baltimore). 2019;98(38):e17219. https://doi.org/10.1097/MD.0000000000017219.
Finianos ES, Yacoub SF, Chammas MF. Ovarian vein thrombosis complicated by pulmonary embolism after cesarean delivery in the presence of a large fibroid: case report and literature review of contributing factors. Case Rep Obstet Gynecol. 2021;2021:6389713.
Tiwary, Manish Kumar; Nair, Abhijit Sukumaran. Challenges in managing postoperative pulmonary embolism after cesarean section. Saudi J Health Sci. 2022;11(1):74–6. https://doi.org/10.4103/sjhs.sjhs_24_22.
Wu YY, Shan TT, Pan XT. Pulmonary embolism after in vitro fertilization and cesarean section: two case reports and brief review of the literature. Int J Womens Health. 2022;14:1489–97.
Zhang J, Yu C, Liu H, Zhu Q. Sudden respiratory and circulatory collapse after cesarean section: amniotic fluid embolism or other reasons? – a case report. BMC Pregnancy Childbirth. 2022;22(1):369.
Zawiślak J, Baczewski K, Targońska S, Stadnik A, Artykiewicz K, Baszak J, et al. Successful emergency surgical pulmonary embolectomy for massive pulmonary embolism after urgent cesarean delivery. Kardiol Pol. 2023;81(2):192–4.
Kobayashi H, Akasaka J, Naruse K, Sado T, Tsunemi T, Niiro E, et al. Comparison of the different definition criteria for the diagnosis of amniotic fluid embolism. J Clin Diagn Res. 2017;11(7):Qc18–qc21.
Ponzio-Klijanienko A, Vincent-Rohfritsch A, Girault A, Le Ray C, Goffinet F, Bonnet MP. Evaluation of the 4 diagnosis criteria proposed by the SMFM and the AFE foundation for amniotic fluid embolism in a monocentric population. J Gynecol Obstet Hum Reprod. 2020;49(9):101821.
Zhang L, Chen Y, Liu W, Wang X, Zhang S, Zhang W, et al. Predictive value of D-dimer and analysis of risk factors in pregnant women with suspected pulmonary embolism after cesarean section. BMC Pulm Med. 2021;21(1):391.
van der Pol LM, Tromeur C, Bistervels IM, Ni Ainle F, van Bemmel T, Bertoletti L, et al. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med. 2019;380(12):1139–49.
Iwamoto T, Hatayama Y, Namba H, Kojima N, Horie T, Yamashita N, et al. Fibrin monomer complex as a potential thrombosis marker related to venous thromboembolism risk in pregnant women. Ann Clin Biochem. 2023;60(4):279–85.
Shayganfar A, Hajiahmadi S, Astaraki M, Ebrahimian S. The assessment of acute pulmonary embolism severity using CT angiography features. Int J Emerg Med. 2020;13(1):15.
Pandey K, Singh A. Amniotic fluid embolism. In: Sharma A, editor. Labour Room Emergencies. Singapore: Springer Singapore; 2020. p. 403–13.
Akazawa M, Nishida M. Thrombolysis with intravenous recombinant tissue plasminogen activator during early postpartum period: a review of the literature. Acta Obstet Gynecol Scand. 2017;96(5):529–35.
Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract Thromb Haemost. 2021;5(5):e12546.
Schutgens REG, Lisman T. Tranexamic acid is not a universal hemostatic agent. HemaSphere. 2021;5(8):e625.
Pacheco LD, Clifton RG, Saade GR, Weiner SJ, Parry S, Thorp JM Jr, et al. Tranexamic acid to prevent obstetrical hemorrhage after cesarean delivery. N Engl J Med. 2023;388(15):1365–75.
Vuong ADB, Pham TH, Pham XTT, et al. Modified one-step conservative uterine surgery (MOSCUS) versus cesarean hysterectomy in the management of placenta accreta spectrum: A single-center retrospective analysis based on 619 Vietnamese pregnant women. Int J Gynaecol Obstet. 2023. https://doi.org/10.1002/ijgo.15220.
Soo Youn Song, Dan Bit Park, Mina Lee, Hyun Jeong Song, Mia Park, You Jin Kim, Byung Hun Kang, Young Bok Ko, Heon Jong Yoo. Obstructive uropathy associated with a Retzius space hematoma following postpartum pulmonary embolism. Clin Exp Obstet Gynecol. 2022;49(5):122. https://doi.org/10.31083/j.ceog4905122.
Yu H, Liu Z, Lu J, Yang X, Yan XX, Mi Y, et al. Lipocalin-2 predicts long-term outcome of normotensive patients with acute pulmonary embolism. Cardiovasc Toxicol. 2020;20(2):101–10.
Rali P, Sacher D, Rivera-Lebron B, Rosovsky R, Elwing JM, Berkowitz J, et al. Interhospital transfer of patients with acute pulmonary embolism: challenges and opportunities. Chest. 2021;160(5):1844–52.
Simcox LE, Ormesher L, Tower C, Greer IA. Pulmonary thrombo-embolism in pregnancy: diagnosis and management. Breathe (Sheff). 2015;11(4):282–9.
Acknowledgements
We thank the patient and her family, who agreed to allow us to publish the clinical data. The authors are also grateful for all colleagues working at the Department of Anesthetist and Resuscitation, Department of High-risk Pregnancy, and Department of Imaging Diagnosis. We thank our colleagues from Cho Ray Hospital for their contributions to patient care. We thank all involved in the care of this patient and those who provided us with great pictures and videos and took such excellent care of the patient, not to mention the surgeons who directly performed the operation.
Funding
No funding was required in the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
A.D.B.V., X.T.N., N.B.T., and Y.O.N.N. were involved in patient care. T.H.P. and V.H.B. were responsible for administrative procedures. D.K.T.L. collected the data and contributed to write a part of original draft. P.N.N. contributed to be responsible for administrative procedures, to receiving information, collecting the data, viewing the literature, mainly writing, editing, and revising the manuscript. A.D.B.V. and P.N.N. contributed equally to this paper and shared the first authorship. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was naturally waived for case reports by the ethics committee of Tu Du Hospital. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Written informed consent was obtained from the participant.
Consent for publication
Written informed consent was obtained from the patient for publication of this study and accompanying images.
Competing interests
The authors declare no competing interests. Phuc Nhon Nguyen was the guarantor of this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary video 1. Sizable thrombus in the pulmonary vascular.
Additional file 2: Supplementary video 2. Sizable thrombus in the subclavian vein.
Additional file 3: Supplementary video 3. Sizable thrombus in the right jugular vein.
Additional file 4: Supplementary video 4. Sizable thrombus in the right ventricular.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Vuong, A.D.B., Pham, T.H., Bui, V.H. et al. Successfully conservative management of the uterus in acute pulmonary embolism during cesarean section for placenta previa: a case report from Tu Du Hospital, Vietnam and literature review. Int J Emerg Med 17, 14 (2024). https://doi.org/10.1186/s12245-024-00587-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12245-024-00587-4