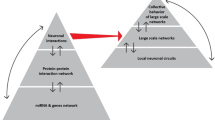
Abstract
Studies of the late 20th century have confirmed the idea that long-term changes in the synaptic system of the human brain form the basis for fixing and maintaining external information. Such phenomena as learning and memory are expressions of these changes at the physiological level. As a result of these studies, the “material” base of these processes was determined as a complex of molecular events that start from the neurotransmitter-synapse interaction and ends with activation of the epigenetic structures of neuronal nucleus. The central event is a coordinated system of signaling pathways, including transduction, transcription, and regulatory (neurotrophic) proteins. Consistent temporal and spatial activations of these structures are the biochemical basis for memory formation and cognitive functions. Synaptic plasticity as a primary mechanism of memory and a multilevel system of signal molecule regulation constitute the essence of organizing different types of memory. The disturbance of neurochemical mechanisms is an initial cause of cognitive dysfunction and psychopathology. At the same time, signal molecules can be considered specific targets for the correction of these states.
Similar content being viewed by others
References
Abel, T., Martin, K.C., Bartsch, D., and Kandel, E.R., Memory suppressor genes: inhibitory constraints on the storage of long-term memory, Science, 1998, vol. 279, pp. 338–341.
Anier, K., Malinovskaja, K., Aonurm-Helm, A., et al., DNA methylation regulates cocaine-induced behavioral sensitization in mice, Neuropsychopharmacology, 2010, vol. 35, pp. 2450–2461.
Anokhin, K.V., Molecular mechanisms of long-term memory, Zh. Vyssh. Nervn. Deyat., 1997, vol. 47, pp. 261–271.
Bartsch, D., Ghirardi, M., Skehel, P.A., et al., Aplysia CREB-2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change, Cell, 1995, vol. 83, pp. 979–992.
Bekinschtein, P., Kent, B.A., Oomen, C.A., et al., BDNF in the dentate gyrus is required for consolidation of “pattern-separated” memories, Cell Rep., 2013, vol. 5, pp. 759–768.
Blanpied, T.A., Kerr, J.M., and Ehlers, M.D., Structural plasticity with preserved topology in the postsynaptic protein network, Proc. Natl. Acad. Sci. U.S.A., 2008, vol. 105, pp. 12587–12592.
Bourne, J.N. and Harris, K.M., Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP, Hippocampus, 2011, vol. 21, pp. 354–373.
Cajal, S.R., La fine structure des centres nerveux, Proc. R. Soc. Lond., 1894, vol. 55, pp. 444–468.
Cajal, S.R., Recuerdos de Mi Vida, Cambridge, MA MIT Press, 1937.
Casadio, A., Martin, K.C., Giustetto, M., et al., A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis, Cell, 1999, vol. 99, pp. 221–237.
Chen, G., Zou, X., Watanabe, H., et al., CREB binding protein is required for both short-term and long-term memory formation, J. Neurosci., 2010, vol. 30, pp. 13066–13077.
Chwang, W.B., Arthur, J.S., Schumacher, A., and Sweatt, J.D., The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation, J. Neurosci., 2007, vol. 27, pp. 12732–12742.
Cingolani, L.A. and Goda, Y., Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy, Nat. Rev. Neurosci., 2008, vol. 9, pp. 344–356.
Collins, M.O., Husi, H., Yu, L., et al., Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome, J. Neurochem., 2006, vol. 97, suppl. 1, pp. 16–23.
Day, J.J. and Sweatt, J.D., Cognitive neuroepigenetics: a role for epigenetic mechanisms in learning and memory, Neurobiol. Learn. Mem., 2011, vol. 96, pp. 2–12.
De Roo, M., Klauser, P., Garcia, P.M., et al., Spine dynamics and synapse remodeling during LTP and memory processes, Prog. Brain Res., 2008, vol. 169, pp. 199–207.
Fischer, A., Sananbenesi, F., Pang, P.T., et al., Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory, Neuron, 2005, vol. 48, pp. 825–838.
Follert, P., Cremer, H., and Beclin, C., MicroRNAs in brain development and function: a matter of flexibility and stability, Front. Mol. Neurosci., 2014, vol. 7, pp. 513.
Fortress, A.M., Schram, S.L., Tuscher, J.J., and Frick, K.M., Canonical Wnt signaling is necessary for object recognition memory consolidation, J. Neurosci., 2013, vol. 33, pp. 12619–12626.
Gomazkov, O.A., Dominant. XX Century, Neirokhimiya, 1999, vol. 16, pp. 145–156.
Gomez-Palacio-Schjetnan, A., and Escobar, M L., Neu-rotrophins and synaptic plasticity, Curr. Topics Behav. Neurosci., 2013, vol. 15, pp. 117–136.
Gomez-Pinilla, F., Zhuang, Y., Feng, J., et al., Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation, Eur. J. Neurosci., 2011, vol. 33, pp. 383–390.
Graff, J., Kim, D., Dobbin, M.M., and Tsai, L.H., Epigenetic regulation of gene expression in physiological and pathological brain processes, Physiol. Rev., 2011, vol. 91, pp. 603–649.
Grayson, D.R., Jia, X., Chen, Y., et al., Reelin promoter hypermethylation in schizophrenia, Proc. Natl. Acad. Sci. U.S.A., 2005, vol. 102, pp. 9341–9346.
Grinkevich, L.N., Epigenetics and the formation of longterm memory, Neurosci. Behav. Physiol., 2014, vol. 44, no. 2, pp. 200–213.
Grinkevich, L.N., Lisachev, P.D., Kharchenko, O.A., and Vasil’ev, G.V., Expression of MAP/ERK kinase cascade corresponds to the ability to develop food aversion in terrestrial snail at different stages of ontogenesis, Brain Res., 2008, vol. 1187, pp. 12–19.
Guan, J.S., Su, S.C., Gao, J., et al., Cdk5 is required for memory function and hippocampal plasticity via the cAMP signaling pathway, PLoS One, 2011, vol. 6, p. e25735.
Hebb, D.O., The Organization of Behavior: A Neuropsychological Theory, New York Wiley, 1949.
Heine, M., Groc, L., Frischknecht, R., et al., Surface mobility of postsynaptic AMPARs tunes synaptic transmission, Science, 2008, vol. 320, pp. 201–205.
Huang, Y., Doherty, J.J., and Dingledine, R., Altered his-tone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus, J. Neurosci., 2002, vol. 22, pp. 8422–8428.
Hut, R.A. and van der Zee, E.A., The cholinergic system, circadian rhythmicity, and time memory, Behav. Brain Res., 2011, vol. 221, pp. 466–480.
Kandel, E.R., The molecular biology of memory storage: a dialogue between genes and synapses, Science, 2001, vol. 294, no. 5544, pp. 1030–1038.
Kandel, E.R., The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB, Mol. Brain, 2012, vol. 5, p. 14.
Kelley, J.B., Anderson, K.L., Altmann, S.L., and Itzhak, Y., Long-term memory of visually cued fear conditioning: roles of the neuronal nitric oxide synthase gene and cyclic AMP response element-binding protein, Neuroscience, 2011, vol. 174, pp. 91–103.
Kim, J.H., McNally, G.P., and Richardson, R., Recovery of fear memories in rats: role of gamma-amino butyric acid (GABA) in infantile amnesia, Behav. Neurosci., 2006, vol. 120, pp. 40–48.
Korzus, E., Rosenfeld, M.G., and Mayford, M.C., BP his-tone acetyl transferase activity is a critical component of memory consolidation, Neuron, 2004, vol. 42, pp. 961–972.
Koshtoyants, Kh.S., Belkovye tela, obmen veshchestv i nervanaya regulyatsiya (Protein Bodies, Metabolism, and Nervous Regulation), Moscow Nauka, 1951.
Kunde, S.A., Rademacher, N., Tzschach, A., et al., Characterization of de novo MAPK10/JNK3 truncation mutations associated with cognitive disorders in two unrelated patients, Hum. Genet., 2013, vol. 132, pp. 461–471.
La Plant, Q., Vialou, V., Covington, H.E., et al., Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens, Nat. Neurosci., 2010, vol. 13, pp. 1137–1143.
Lashley, K., In search of the engram, Soc. Exp. Biol. Symp., 1950, vol. 4, pp. 454–482.
Levenson J.M., O’ Riordan K.J., Brown K.D., et al., Regulation of histone acetylation during memory formation in the hippocampus, J. Biol. Chem., 2004, vol. 279, pp. 40545–40559.
Levenson J.M., Roth T.L., Lubin F.D., et al., Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus, J. Biol. Chem., 2006, vol. 281, pp. 15763–15773.
Levi-Montalcini, R., The nerve growth factor: thirty-five years later, EMBO J., 1987, vol. 6, pp. 1145–1154.
Loewi, O., Über humorale Übertragbarkeit der Herzner-venwirkung, PflügersArch. Ges. Physiol., 1921, vol. 189, pp. 239–242.
Lu, B., Nagappan, G., and Lu, Y., BDNF and synaptic plasticity, cognitive function and dysfunction, Handb. Exp. Pharmacol., 2014, vol. 220, pp. 223–250.
Maffioletti, E., Tardito, D., Gennarelli, M., and Bocchio-Chiavetto, L., Micro spies from the brain to the periphery: new clues from studies on microRNAs in neuropsychiatric disorders, Front. Cell Neurosci., 2014, vol. 8, p. 75.
Makkar, S.R., Zhang, S.Q., and Cranney, J., Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory, Neuropsychopharmacology, 2010, vol. 35, pp. 1625–1652.
Mamiya, N., Fukushima, H., Suzuki, A., et al., Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory, J. Neurosci., 2009, vol. 29, pp. 402–413.
Michan, S., Li, Y., Chou, M.M., et al., SIRT1 is essential for normal cognitive function and synaptic plasticity, J. Neurosci., 2010, vol. 30, pp. 9695–9707.
Mikaelsson, M.A. and Miller, C.A., The path to epigenetic treatment of memory disorders, Neurobiol. Learn. Mem., 2011, vol. 96, pp. 13–18.
Moncada, D., Ballarini, F., Martinez, M.C., et al., Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation, Proc. Natl. Acad. Sci. U.S.A., 2011, vol. 108, pp. 12931–12936.
Morris, R.G., Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging, and schemas, Eur. J. Neurosci., 2006, vol. 23, pp. 2829–2846.
Morris, K.A. and Gold, P.E., Age-related impairments in memory and in CREB and pCREB expression in hippocampus and amygdala following inhibitory avoidance training, Mech. Ageing Dev., 2012, vol. 133, pp. 291–299.
Muller, D., Toni, N., and Buchs, P.A., Spine changes associated with long-term potentiation, Hippocampus, 2000, vol. 10, pp. 596–604.
Murakoshi, H. and Yasuda, R., Postsynaptic signaling during plasticity of dendritic spines, Trends Neurosci., 2012, vol. 35, pp. 135–143.
Nagerl, U.V., Eberhorn, N., Cambridge, S.B., and Bonho-effer, T., Bidirectional activity-dependent morphological plasticity in hippocampal neurons, Neuron, 2004, vol. 44, pp. 759–767.
Nakazawa, T., Kuriu, T., Tezuka, T., et al., Regulation of dendritic spine morphology by an NMDA receptor-associated Rho GTP-ase-activating protein, p250GAP, J. Neurochem., 2008, vol. 105, pp. 1384–1393.
Nelson, E.D., Kavalali, E.T., and Monteggia, L.M., Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation, J. Neurosci., 2008, vol. 28, pp. 395–406.
O’Riordan, K.J., Huang, I.C., Pizzi, M., et al., Regulation of nuclear factor kappaB in the hippocampus by group I metabotropic glutamate receptors, J. Neurosci., 2006, vol. 26, pp. 4870–4879.
Park, P., Volianskis, A., and Sanderson, T.M., NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation, Philos. Trans. R. Soc., B, 2013, vol. 369, no. 1633. https://www.ncbi.nlm.nih.gov/pubmed/24298134
Petrini, E.M., Lu, J., Cognet, L., et al., Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation, Neuron, 2009, vol. 63, pp. 92–105.
Puckett, R.E. and Lubin, F.D., Epigenetic mechanisms in experience-driven memory formation and behavior, Epigenomics, 2011, vol. 3, pp. 649–664.
Rao, P., Benito, E., and Fischer, A., MicroRNAs as biomarkers for CNS disease, Front. Mol. Neurosci., 2013, vol. 6, p. 39.
Reichardt, L.F., Neurotrophin-regulated signaling pathways, Philos. Trans. R. Soc., B, 2006, vol. 361(1473), pp. 1545–1564.
Roth, T.L., Lubin, F.D., Funk, A.J., and Sweatt, J.D., Lasting epigenetic influence of early-life adversity on the BDNF gene, Biol. Psychiatry, 2009, vol. 65, pp. 760–769.
Roth, T.L. and Sweatt, J.D., Regulation of chromatin structure in memory formation, Curr. Opin. Neurobiol., 2009, vol. 19, pp. 336–342.
Rouaux, C., Loeffler, J.P., and Boutillier, A.L., Targeting CREB-binding protein (CBP) loss of function as a therapeutic strategy in neurological disorders, Biochem. Pharmacol., 2004, vol. 68, pp. 1157–1164.
Segal, M., Dendritic spines and long-term plasticity, Nat. Rev. Neurosci., 2005, vol. 6, pp. 277–284.
Sheng, M. and Hoogenraad, C.C., The postsynaptic architecture of excitatory synapses: a more quantitative view, Annu. Rev. Biochem., 2007, vol. 76, pp. 823–847.
Sherrington, C.S., The Integrative Action of the Nervous System, New Haven Yale Univ. Press, 1906, p. 18.
Sherry, J.M. and Crowe, S.F., Inhibition of cyclin-depen-dent kinase 5 by roscovitine impairs memory consolidation and reconsolidation in the day-old chick, Pharmacol. Biochem. Behav., 2008, vol. 91, pp. 59–66.
Shevchenko, K.G., Danilova, A.B., and Grinkevich, L.N., Post-translational modification of histone H3 at consolidation and re-consolidation of memory in Helix mollusk, Vestn. Vavilov. O-va Genet. Selekts., 2009, vol. 13, pp. 723–729.
Si, J., Yang, J., Xue, L., et al., Activation of NF-kB in baso-lateral amygdala is required for memory reconsolidation in auditory fear conditioning, PLoS One, 2012, vol. 7, p. e43973.
Steiner, P., Higley, M.J., Xu, W, et al., Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity, Neuron, 2008, vol. 60, pp. 788–802.
Tsankova, N.M., Berton, O., Renthal, W., et al., Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action, Nat. Neurosci., 2006, vol. 9, pp. 519–525.
Valnegri, P., Sala, C., and Passafaro, M., Synaptic dysfunction and intellectual disability, Adv. Exp. Med. Biol., 2012, vol. 970, pp. 433–449.
Valor, L.M., Viosca, J., Lopez-Atalaya, J.P., and Barco, A., Lysine acetyltransferases CBP and p300 as therapeutic targets in cognitive and neurodegenerative disorders, Curr. Pharm. Des., 2013, vol. 19, pp. 5051–5064.
Whittle, N. and Singewald, N., HDAC inhibitors as cognitive enhancers in fear, anxiety and trauma therapy: where do we stand? Biochem. Soc. Trans., 2014, vol. 42, pp. 569–581.
Yoon, K.J., Lee, H.R., Jo, Y.S., et al., Mind bomb-1 is an essential modulator of long-term memory and synaptic plasticity via the Notch signaling pathway, Mol. Brain, 2012, vol. 5, pp. 40–47.
Zhang, J., Little, C.J., Tremmel, D.M., et al., Notch-inducible hyperphosphorylated CREB and its ultradian oscillation in long-term memory formation, J. Neurosci., 2013, vol. 33, pp. 12825–12834.
Zhou, Q., Homma, K.J., and Poo, M.M., Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses, Neuron, 2004, vol. 44, pp. 749–757.
Zovkic, I.B., Guzman-Karlsson, M.C., and Sweatt, J.D., Epigenetic regulation of memory formation and maintenance, Learn. Mem., 2013, vol. 20, pp. 61–74.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.A. Gomazkov, 2014, published in Uspekhi Sovremennoi Biologii, 2014, Vol. 134, No. 6, pp. 545–562.
Rights and permissions
About this article
Cite this article
Gomazkov, O.A. How do signaling molecules organize higher brain functions?. Biol Bull Rev 5, 281–295 (2015). https://doi.org/10.1134/S2079086415040015
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086415040015