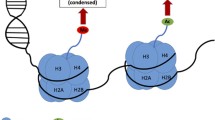
The most urgent problem in neurobiology is the search for the mechanisms underlying long-term memory. Gene expression has been shown to be needed for long-term memory to form. Which genes are expressed and how their expression is regulated are subject to intense investigation. The task is complicated by the multicomponent nature of the systems regulating the genome. A number of DNA-binding transcription factors involved in regulating gene expression in learning have now been identified. However, activation of these factors alone is insufficient to induce expression; modification (remodeling) of chromatin is also required. Chromatin remodeling processes are epigenetic. The most important roles in chromatin remodeling are played by the processes of phosphorylation, acetylation, and methylation of histones and methylation of DNA. Impairments to these processes lead to the inability to form long-term memory. This review addresses the role of transcription factors and chromatin modifications in the formation of long-term memory in invertebrates and vertebrates and assesses the potential for improving memory by modifying epigenetic mechanisms.
Similar content being viewed by others
References
K. V. Anokhin, “Molecular scenarios for the consolidation of longterm memory,” Zh. Vyssh. Nerv. Deyat., 47, No. 2, 261–279 (1997).
V. L. Bianki and E. B. Filippova, Evolution of Functional Asymmetry of the Brain [in Russian], Nauka, Leningrad (1987), pp. 304–352.
L. N. Grinkevich, P. D. Lisachev, and T. I. Merkulova, “Formation of AP-1 transcription factors during learning in Helix,” Ros. Fiziol. Zh., 87, No. 6, 762–773 (2001).
L. N. Grinkevich and G. V. Vasil’ev, “Possible molecular-cellular mechanisms for the regulation of gene expression during learning,” Ros. Fiziol. Zh., 85, No. 1, 48–66 (1999).
L. N. Grinkevich, P. D. Lisachev, K. A. Baranova, and O. A. Kharchenko, “Comparative analysis of the activation of MAPK/ERK kinases in the CNS of animals with different learning abilities,” Ros. Fiziol. Zh., 92, No. 6, 536–545 (2006).
A. B. Danilova, P. D. Lisachev, and L. N. Grinkevich, “Comparative studies of protein acetylation in the CNS of adult and juvenile Helix during the formation of long-term memory,” Info. Vestn. VOGiS, 14, No. 2, 312–319 (2010).
V. A. Dyatlov, “The role of calcium ions in the processes of modulation of neuron responses to application of acetylcholine by serotonin in the common snail,” Neirofiziologiya, 20, No. 5, 666–671 (1988).
V. L. Karpov, “What determines the fate of a gene?” Priroda, No. 3, 34–43 (2005).
A. A. Pendina, V. V. Grinkevich, T. V. Kuznetsova, and V. S. Baranov, “DNA methylation – a universal mechanism regulating gene activity,” Ekol. Genet., 2, No. 3, 27–36 (2004).
N. B. Salimova, I. Miloshevich, and R. M. Salimov, “The actions of 5,6-hydroxytryptamine on behavior in the snail labyrinth,” Zh. Vyssh. Nerv. Deyat., 34, No. 5, 941–947 (1984).
K. G. Shevchenko, A. B. Danilova, and L. N. Grinkevich, “Posttranslational modification of histone H3 on memory consolidation and reconsolidation in the mollusk Helix,” Info. Vestn. VOGiS, 13, No. 4, 723–730 (2009).
T. Abel and R. S. Zukin, “Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders,” Curr. Opin. Pharmacol., 8, No. 1, 57–64 (2008).
S. Akbarian and H. S. Huang, “Epigenetic regulation in human brain – focus on histone lysine methylation,” Biol. Psychiatry, 65, No. 3, 198–203 (2009).
J. M. Alarcon, G. Malleret, K. Touzani, et al., “Chromatin acetylation, memory, and LTP are impaired in CBP +/– mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration,” Neuron, 42, No. 6, 947–959 (2004).
C. M. Alberini, “Transcription factors in long-term memory and synaptic plasticity,” Physiol. Rev., 89, No. 1, 121–145 (2009).
C. M. Alberini, M. Ghirardi, R. Metz, and E. R. Kandel, “C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia,” Cell, 76, 1099–1114 (1994).
C. M. Atkins, J. S. Selcher, J. J. Petraitis, et al., “The MAPK cascade is required for mammalian associative learning,” Nat. Neurosci., 1, No. 7, 602–609 (1998).
P. M. Balaban, “Cellular mechanisms of behavioral plasticity in terrestrial snail,” Neurosci. Biobehav. Rev., 26, 597–630 (2002).
A. Barco and H. Marie, “Genetic approaches to investigate the role of CREB in neuronal plasticity and memory,” Mol. Neurobiol., 44, No. 3, 330–349 (2011).
D. Bartsch, M. Ghirardi, P. Skehal, et al., “Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change,” Cell, 83, No. 2, 979–992 (1995).
S. L. Berger, “The complex language of chromatin regulation during transcription,” Nature, 447, 407–412 (2007).
D. E. Berman, S. Hazvi, K. Rosenblum, et al., “Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat,” J. Neurosci., 18, 10037–10044 (1998).
S. Blum, A. N. Moore, F. R. Adams, and P. K. Dash, “A mitogen-activated protein kinase cascade in CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory,” J. Neurosci., 19, 3535–3544 (1999).
T. W. Bredy, H. Wu, C. Crego, et al., “Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear,” Learn. Mem., 14, No. 4, 268–276 (2007).
T. J. Carew, “Molecular enhancement of memory formation,” Neuron, 16, 5–8 (1996).
D. Chakravarti, V. J. La Monte, M. C. Nelson, et al., “Role of CBP/p300 in nuclear receptor signaling,” Nature, 383, 99–103 (1996).
Y. Chandramohan, S. K. Droste, J. S. Arthur, and J. M. Reul, “The forced swimming-induced behavioral immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signaling pathway,” Eur. J. Neurosci., 27, No. 10, 2701–2713 (2008).
P. Cheung, C. D. Allis, and P. Sassone-Corsi, “Signaling to chromatin through histone modifications,” Cell, 103, 263–271 (2000).
P. Cheung, K. G. Tanner,W. L. Cheung, et al., “Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation,” Mol. Cell., 5, No. 6, 905–915 (2000).
J. C. Chrivia, R. P. S. Kwok, N. Lamb, et al., “Phosphorylated CREB binds specifically to the nuclear protein CBP,” Nature, 365, 855–859 (1993).
W. B. Chwang, K. J. O’Riordan, J. M. Levenson, and J. D. Sweatt, “ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning,” Learn. Mem., 13, 322–328 (2006).
W. B. Chwang, J. S. Arthur, A. Schumacher, and J. S. Sweatt, “The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation,” J. Neurosci., 27, No. 46, 12732–12742 (2007).
T. Crow, J.-J. Xue-Bian, V. Siddiqi, and J. T. Neary, “Serotonin activation of ERK pathway in Hermissenda: contribution of calciumdependent protein kinase C,” J. Neurochem., 78, 358–364 (2001).
A. B. Danilova, O. A. Kharchenko, K. G. Shevchenko, and L. N. Grinkevich, “Histone H3 acetylation is asymmetrically induced upon learning in identified neurons of the food aversion network in the mollusk Helix lucorum,” Front. Behav. Neurosci., 4, 180, 1–7 (2010).
P. K. Dash, S. A. Orsi, and A. N. Moore, “Sequestration of serum response factor in the hippocampus impairs long-term spatial memory,” J. Neurochem., 93, 268–278 (2005).
A. J. de Ruijter, A. H. van Gennip, H. N. Caron, et al., “Histone deacetylase (HDACs): characterization of the classical HDAC family,” Biochem. J., 370, 737–749 (2003).
G. P. Delcuve, M. Rastegar, and J. R. Davie, “Epigenetic control,” J. Cell. Physiol., 219, No. 2, 243–250 (2009).
S. Duvarci, K. Nader, and J. E. LeDoux, “Activation of extracellular signal-regulated kinase-mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning,” Eur. J. Neurosci., 21, No. 1, 283–289 (2005).
H. Einat, P. Yuan, T. D. Gould, et al., “The role of the extracellular signal-regulated kinase signaling pathway in mood modulation,” J. Neurosci., 23, No. 19, 7311–7316 (2003).
G. Faraco, T. Pancani, L. Formentini, et al., “Pharmacological inhibition of histone deacetylase by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain,” Mol. Pharmacol., 70, No. 6, 1876–1884 (2006).
V. Feld, B. Dimant, A. Delorenzi, et al., “Phosphorylation of extranuclear ERK/MAPK is required for long-term memory consolidation in the crab Chasmognathus,” Behav. Brain Res., 158, 251–261 (2005).
R. J. Ferrante, J. K. Kubilus, J. Lee, et al., “Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice,” J. Neurosci., 23, No. 28, 9418–9427 (2003).
R. D. Fields, F. Eshete, B. Stevens, and K. Itoh, “Action potentialdependent regulation of gene expression: temporal specificity in Ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling,” J. Neurosci., 17, No. 19, 7252–7266 (1997).
A. Fischer, F. Sananbenesi, X. Wang, et al., “Recovery of learning and memory is associated with chromatin remodeling,” Nature, 447, No. 7141, 178–182 (2007).
L. Formisano, K. M. Noh, T. Miyawaki, et al., “Ischemic insults promote epigenetic reprogramming of mu opioid receptor expression in hippocampal neurons,” Proc. Natl. Acad. Sci. USA, 104, No. 10, 4170–4175 (2007).
G. Gardian, S. E. Browne, D. K. Choi, et al., “Neuroprotective effects of phenylbutyrate in the N171-82Q transgenic mouse model of Huntington’s disease,” J. Biol. Chem., 280, No. 1, 556–563 (2005).
L. N. Grinkevich, “Formation of C/EBP transcription factors and possible pathways for controlling their activity during learning in Helix,” Neurosci. Behav. Physiol., 32, No. 1, 33–39 (2002).
L. N. Grinkevich, P. D. Lisachev, O. A. Kharchenko, et al., “Expression of MAP/ERK kinase cascade corresponds to the ability to develop food aversion in terrestrial snail at different stages of ontogenesis,” Brain Res., 1187, 12–19 (2008).
Z. Guan, M. Giustetto, S. Lomvardas, et al., “Integration of longterm memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure,” Cell, 111, No. 4, 483–493 (2002).
S. Gupta, S. Y. Kim, S. Artis, et al., “Histone methylation regulates memory formation,” J. Neurosci., 30, No. 10, 3589–3599 (2010).
T. Herdegen and J. D. Leah, “Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos, and Krox, and CREB/ARF proteins,” Brain Res. Rev., 28, No. 3, 370–490 (1998).
O. Hobert, R. J. Johnston, Jr., and S. Chang, “Left-right asymmetry in the nervous system: the Caenorhabditis elegans model,” Nat. Rev. Neurosci., 3, No. 8, 629–640 (2002).
S. C. Hu, J. Chrivia, and A. Ghosh, “Regulation of CBP-mediated transcription by neuronal calcium signaling,” Neuron, 22, No. 4, 799–808 (1999).
R. Janknecht and T. Hunter, “A growing coactivator network,” Nature, 383, No. 6595, 22–23 (1996).
L. Kaczmarek and A. Chandhuri, “Sensory regulation of immediate early gene expression in mammalian visual cortex: implications for functional mapping and neural plasticity,” Brain Res. Rev., 23, 237–256 (1997).
B. Kaminska, L. Kaczmarek, S. Zangenehpour, and A. Chandhuri, “Rapid phosphorylation of Elk-1 transcription factor and activation of MAP kinase signal transduction pathways in response to visual stimulation,” Mol. Cell. Neurosci., 13, 405–414 (1999).
E. R. Kandel, “The molecular biology of memory storage: a dialogue between genes and synapses,” Science, 294, No. 5544, 1030–1038 (2001).
D. R. Kaplan and F. D. Miller, “Neurotrophin signal transduction in the nervous system,” Curr. Opin. Neurobiol., 10, No. 3, 381–391 (2000).
M. Karin, Z. Liu, and E. Zandi, “AP-1 function and regulation,” Curr. Opin. Cell. Biol., 9, No. 2, 240–246 (1997).
A. Kelly, S. Laroche, and S. Davis, “Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation memory,” J. Neurosci., 23, No. 12, 5354–5360 (2003).
O. A. Kharchenko,V. V. Grinkevich, O. V. Vorobiova, and L. N. Grinkevich, “Learning-induced lateralized activation of the MAPK/ERK cascade in identified neurons of the food-aversion network in the mollusk Helix lucorum,” Neurobiol. Learn. Mem., 94, No. 2, 158–166 (2010).
J. M. Kornhauser and M. E. Greenberg, “A kinase to remember: dual roles for MAP kinase in long-term memory,” Neuron, 18, No. 6, 839–842 (1997).
E. Korzus, M. G. Rosenfeld, and M. Mayford, “CBP histone acetyltransferase activity is a critical component of memory consolidation,” Neuron, 42, No. 6, 961–972 (2004).
S. V. Kyosseva, “Mitogen-activated protein kinase signaling,” Int. Rev. Neurobiol., 59, 201–220 (2004).
J. L. Lee, P. De Ciano, B. J. Everitt, and K. L. Thomas, “Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior,” Neuron, 47, No. 6, 795–801 (2005).
E. Lesburgueres, O. L. Gobbo, S. Alaux-Cantin, et al., “Early tagging of cortical networks is required for the formation of enduring associative memory,” Science, 331, No. 6019, 924–928 (2011).
P. Letzkus, N. Boeddeker, J. T. Wood, et al., “Lateralization of visual learning in the honeybee,” Biol. Lett., 4, No. 1, 16–18 (2008).
J. M. Levenson, K. J. O’Riordan, K. D. Brown, et al., “Regulation of histone acetylation during memory formation in the hippocampus,” J. Biol. Chem., 279, 40545–40559 (2004).
J. M. Levenson and J. D. Sweatt, “Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation,” Cell. Mol. Life Sci., 63, 1009–1016 (2006).
Q. Lu, A. E. Hutchins, C. M. Doyle, et al., “Acetylation of cAMPresponsive element-binding protein (CREB) by CREB-binding protein enhances CREB-dependent transcription,” J. Biol. Chem., 278, No. 18, 15727–15734 (2003).
F. D. Lubin and J. D. Sweatt, “The IkB kinase regulates chromatin structure during reconsolidation of conditioned fear memories,” Neuron, 55, No. 6, 942–957 (2007).
F. D. Lubin, “Epigenetic gene regulation in the adult mammalian brain: multiple roles in memory formation,” Neurobiol. Learn. Mem., 96, No. 1, 68–78 (2011).
H. Martin, M. Flandez, C. Nombela, and M. Molina, “Protein phosphatases in MAPK signalling: we keep learning from yeast,” Mol. Microbiol., 58, No. 1, 6–16 (2005).
K. C. Martin, D. Michael, J. C. Rose, et al., “MAP kinase translocates into the nucleus of the presynaptic cell and is required for longterm facilitation in Aplysia,” Neuron, 18, 899–912 (1997).
K. J. McManus and M. J. Hendzel, “CBP, a transcriptional coactivator and acetyltransferase,” Biochem. Cell. Biol., 79, No. 3, 253–266 (2001).
B. Mellstrom, J. R. Naranjo, N. S. Folkes, et al., “Transcriptional response to cAMP in brain: specific distribution and induction of CREM antagonists,” Neuron, 10, 655–665 (1993).
K. Merienne, S. Pannetier, A. Harel-Bellan, and P. Sassone-Corsi, “Mitogen-regulated RSK2-CBP interaction controls their kinase and acetylase activities,” Mol. Cell. Biol., 21, No. 20, 7089–7096 (2001).
C. A. Miller, S. L. Campbell and J. D. Sweatt, “DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity,” Neurobiol. Learn. Mem., 89, 599–603 (2008).
M. S. Monsey, K. T. Ota, I. F. Akingbade, et al., “Epigenetic alterations are critical for fear memory consolidation and synaptic plasticity in the lateral amygdala,” PLoS One, 6, No. 5, 1–13 (2011).
K. Nader and O. Hardt, “A single standard for memory: the case for reconsolidation,” Nature Rev. Neurosci., 10, No. 3, 224–234 (2009).
P. C. Orban, P. F. Chapman, and R. Brambilla, “Is the Ras-MAPK signalling pathway necessary for long-term memory formation?” Trends Neurosci., 22, No. 1, 38–44 (1999).
L. C. Ou and P. W. Gean, “Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory,” Mol. Pharmacol., 72, No. 2, 350–358 (2007).
A. Pascual, K. L. Huang, J. Neveu, and T. Preat, “Neuroanatomy: brain asymmetry and long-term memory,” Nature, 427, No. 6975, 605–606 (2004).
N. D. Perkins, L. K. Felzien, J. C. Betts, et al., “Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator,” Science, 275, No. 5299, 523–527 (1997).
C. L. Peterson and M. A. Laniel, “Histones and histone modifications,” Curr. Biol., 14, No. 14, 546–551 (2004).
F. Petrij, R. H. Giles, H. G. Dauwerse, et al., “Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP,” Nature, 376, No. 6538, 348–351 (1995).
L. J. Rogers and G. Vallortigara, “From antenna to antenna: Lateral shift of olfactory memory recall by honeybees,” PLoS One, 3, No. 6, e2340 (2008).
F. Sananbenesi, A. Fischer, C. Schrick, et al., “Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor2: a possible link between stress and fear memory,” J. Neurosci., 23, No. 36, 11436–11443 (2003).
P. Sassone-Corsi, C. A. Mizzen, P. Cheung, et al., “Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3,” Science, 285, No. 5429, 886–891 (1999).
F. A. Schroeder, C. L. Lin, W. E. Crusio, and S. Akbarian, “Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse,” Biol. Psychiatry, 62, No. 1, 55–64 (2007).
V. Sgambato, P. Vanhoutte, C. Pages, et al., “In vivo expression and regulation of Elk-1, a target of the extracellular-regulated kinase signaling pathway, in the adult rat brain,” J. Neurosci., 18, No. 1, 214–226 (1998).
S. Shen and P. Casaccia-Bonnefil, “Post-translational modifications of nucleosomal histones in oligodendrocyte lineage cells in development and disease,” J. Mol. Neurosci., 35, No. 1, 13–22 (2008).
A. Soloaga, S. Thomson, G. R. Wiggin, et al., “MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14,” EMBO J., 22, No. 11, 2788–2797 (2003).
S. Spange, T. Wagner, T. Heinzel, and O. H. Kramer, “Acetylation of non-histone proteins modulates cellular signaling at multiple levels,” Int. J. Biochem. Cell. Biol., 41, 185–198 (2009).
A. Stipanovich, E. Valjent, M. Matamales, et al., “A phosphatase cascade by which rewarding stimuli control nucleosomal response,” Nature, 453, 879–885 (2008).
B. D. Strahl and C. D. Allis, “The language of covalent histone modifications,” Nature, 403, No. 6765, 41–45 (2000).
H. Suzuki, T. R. Thiele, S. Faumont, et al., “Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis,” Nature, 454, No. 7200, 114–117 (2008).
M. W. Swank and J. D. Sweatt, “Increased histone acetyltransferase and lysine acetyltransferase activity and biphasic activation of the ERK/RSK cascade in insular cortex during novel taste learning,” J. Neurosci., 21, 3383–3391 (2001).
J. D. Sweatt, “Experience-dependent epigenetic modifications in the central nervous system,” Biol. Psychiatry, 65, 191–197 (2009).
G. M. Thomas and R. L. Huganir, “MAPK cascade signaling and synaptic plasticity,” Nat. Rev. Neurosci., 5, No. 3, 173–183 (2004).
R. Treisman, “Journey to the surface of the cell: fos regulation and the SRE,” EMBO J., 14, 4905–4913 (1995).
N. C. Tronson and J. C. Taylor, “Molecular mechanisms of memory reconsolidation,” Nature Rev. Neurosci., 8, 262–275 (2007).
N. M. Tsankova, O. Berton, W. Renthal, et al., “Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action,” Nat. Neurosci., 9, No. 4, 519–525 (2006).
M. A. Wood, J. D. Hawk, and T. Abel, “Combinatorial chromatin modifications and memory storage: A code for memory,” Learn. Mem., 13, 241–244 (2006).
J. Xing, D. D. Ginty, and M. E. Greenberg, “Coupling of the RASMAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase,” Science, 273, No. 5277, 959–963 (1996).
W. Xu, H. Chen, K. Du, et al., “A transcriptional switch mediated by cofactor methylation,” Science, 294, No. 5551, 2507–2511 (2001).
I. S. Zakharov and P. M. Balaban, “Neural mechanisms of agedependent changes in avoidance behaviour of the snail Helix lucorum,” Neuroscience, 23, No. 2, 721–729 (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 98, No. 5, pp. 553–574, May, 2012.
Rights and permissions
About this article
Cite this article
Grinkevich, L.N. Epigenetics and the Formation of Long-Term Memory. Neurosci Behav Physi 44, 200–213 (2014). https://doi.org/10.1007/s11055-014-9897-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-014-9897-2