Abstract
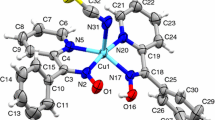
The one-pot synthesis of the Cu(II) complex with hard to access symmetric bis-azoligand is carried out by the reaction of 4,5-dimethyl-1,2-phenylenediamine (Dmpda) with the [Cu2(Piv)4(HPiv)2] complex (Piv is the pivalate anion) in the presence of atmospheric oxygen. The amine form of the ligand takes place and the bis-diiminosemiquinone biradical redox form of the ligand does not take place in the [CuLazo] complex as unambiguously shown by the data of X-ray structure analysis (CIF files CCDC nos. 1877582 (I · DMF) and 1877583 (II), ESR spectroscopy, SQUID magnetometry, and electrochemical and quantum chemical studies. The coordination polymer [Cu2(Piv)4(Dmpda)]n in which the Dmpda molecules perform the bridging function is formed in the same reaction mixture under reduced air pressure.









Similar content being viewed by others
REFERENCES
Balch, A.L. and Holm, R.H., J. Am. Chem. Soc., 1966, vol. 88, p. 5201.
Eremenko, I.L., Nefedov, S.E., Sidorov, A.A., et al., J. Organomet. Chem., 1998, vol. 551, p. 171.
Sidorov, A.A., Danilov, P.V., Nefedov, S.E., et al., Russ. J. Inorg. Chem., 1998, vol. 43, p. 846.
Fomina, I.G., Talismanov, S.S., Sidorov, A.A., et al., Russ. Chem. Bull., 2001, vol. 50, p. 515.
Herebian, D., Bothe, E., Neese, F., et al., J. Am. Chem. Soc., 2003, vol. 125, p. 9116.
Khusniyarov, M.M., Harms, K., Burghaus, O., et al., Dalton Trans., 2008, p. 1355.
Stokes, F.A., Kloo, L., Lv, Y., et al., Chem. Commun., 2012, vol. 48, p. 11298.
Mederos, A., Dominguez, S., Hernandez-Molina, R., et al., Coord. Chem. Rev., 1999, vols. 193−195, p. 913.
Poddel’sky, A.I., Cherkasov, V.K., and Abakumov, G.A., Coord. Chem. Rev., 2009, vol. 253, p. 291.
Olivos Suarez, A.I., Lyaskovskyy, V., Reek, J.N.H., et al., Angew. Chem., Int. Ed. Engl., 2013, vol. 52, p. 12510.
Sidorov, A.A., Fomina, I.G., Nesterov, V.V., et al., Russ. Chem. Bull., 1999, vol. 48, no. 3, p. 573.
Leconte, N., Ciccione, J., Gellon, G., et al., Chem. Commun., 2014, vol. 50, p. 1918.
Ciccione, J., Leconte, N., Luneau, D., et al., Inorg. Chem., 2016, vol. 55, p. 649.
Talismanova, M.O., Fomina, I.G., Sidorov, A.A., et al., Russ. Chem. Bull., 2003, vol. 52, no. 12, p. 2706.
Fomina, I.G., Sidorov, A.A., Aleksandrov, G.G., et al., Russ. Chem. Bull., 2002, vol. 51, no. 8, p. 1581.
Malkov, A.E., Aleksandrov, G.G., Ikorskii, V.N., et al., Russ. J. Coord. Chem., 2001, vol. 27, no. 9, p. 636.
Malkov, A.E., Sidorov, A.A., Aleksandrov, G.G., et al., Russ. Chem. Bull., 2003, vol. 52, no. 3, p. 710.
Eremenko, I.L., Malkov, A.E., Sidorov, A.A., et al., Mendeleev Commun., 2003, vol. 13, no. 1, p. 10.
Troyanov, S.I., Il’ina, E.G., Dunaeva, K.M., Koord. Khim., 1991, vol. 17, no. 12, p. 1692.
Denisova, T.O., Amel’chenkova, E.V., Pruss, I.V., et al., Russ. J. Inorg. Chem., 2006, vol. 51, no. 7, p. 1020.
SMART (control) and SAINT (integration) Software. Version 5.0, Madison: Bruker AXS Inc., 1997.
Sheldrick, G.M., SADABS. Program for Scanning and Correction of Area Detector Data, Göttinngen: Univ. of Göttinngen, 2004.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, vol. 64, no. 1, p. 112.
Rakitin, Yu.V. and Kalinnikov, V.T., Sovremennaya magnetokhimiya (Modern Magnetochemistry), St. Petersburg: Nauka, 1994.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., et al., Gaussian 09 (revision E.01), Wallingford: Gaussian, Inc., 2013.
Starikov, A.G., Minyaev, R.M., and Minkin, V.I., J. Mol. Struct.: THEOCHEM, 2009, vol. 895, nos. 1−3, p. 138.
Chemcraft. Version 1.7. 2013. http://www.chemcraftprog.com.
Reuter, R., Hostettler, N., Neuburger, M., and Wegner, H.A., Eur. J. Org. Chem., 2009, vol. 2009, p. 5647.
Hamon, F., Djedaini-Pilard, F., Barbot, F., and Len, C., Tetrahedron, 2009, vol. 65, p. 10105.
Bellotto, S., Reuter, R., Heinis, C., and Wegner, H.A., Org. Chem., 2011, vol. 76, p. 9826.
Kang, H.-M., Kim, H.-Y., Jung, J.-W., and Cho, C.G., J. Org. Chem., 2007, vol. 72, p. 679.
Mathews, M. and Tamaoki, N., J. Am. Chem. Soc., 2008, vol. 130, p. 11409.
Takaishi, K., Kawamoto, M., Muranaka, A., and Uchiyama, M., Org. Lett., 2012, vol. 14, no. 13, p. 3252.
Kerner, L., Kickova, A., Filo, J., et al., J. Phys. Chem. A, 2015, vol. 119, p. 8588.
Lu, J., Xia, A., Zhou, N., et al., Chem.-Eur. J., 2015, vol. 21, p. 2324.
Allen, F.H., Kennard, O., Watson, D.G., et al., Perkin Trans. II., 1987, p. S1.
Adams, H., Bucknall, R.M., Fenton, D.E., et al., Polyhedron, 1998, vol. 17, nos. 23−24, p. 4169.
Dhara, P.K., Pramanik, S., Lu, T.-H., et al., Polyhedron, 2004, vol. 23, p. 2457.
Jarvis, J.A.J., Acta Crystallogr., 1961, vol. 14, no. 9, p. 961.
Benaouida, M.A., Benosmane, A., Bouguerria, H., et al., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2013, vol. 69, p. m405.
Abrahams, B.F., Egan, S.J., and Robson, R., J. Am. Chem. Soc., 1999, vol. 121, no. 14, p. 3535.
Sarkar, S., Dhara, P.K., Nethaji, M., and Chattopadhyay, P., J. Coord. Chem., 2009, vol. 62, no. 5, p. 817.
Tai, W.-J., Li, C.-H., Li, C.-Y., and Ko, B.-T., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, vol. 66, p. m1315.
Burlov, A.S., Uraev, A.I., Lyssenko, K.A., et al., Russ. J. Coord. Chem., 2006, vol. 32, no. 9, p. 686. https://doi.org/10.1134/S1070328406090119
Speier, G., Csihony, J., Whalen, A.M., and Pierpont, C.G., Inorg. Chem., 1996, vol. 35, p. 3519.
Burlov, A.S., Uraev, A.I., Matuev, P.V., et al., Russ. J. Coord. Chem., 2008, vol. 34, no. 12, p. 904. https://doi.org/10.1134/S1070328408120063
Bill, E., Bothe, E., Chaudhuri, P., et al., Chem.-Eur. J., 2005, vol. 11, p. 204.
Ng, S.W., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, vol. 65, p. o1069.
Stibrany, R.T. and Potenza, J.A., CSD Commun., 2012 (Private Communication; refcode GOGWEG, CCDC 893911).
Rakitin, Yu.V., Larin, G.M., and Minin, V.V. Interpretatsiya spektrov EPR koordinatsionnykh soedinenii (Interpretation of the ESR Spectra of Coordination Compounds), Moscow: Nauka, 1993.
Lebedev, Ya.S. and Muromtsev, V.I., EPR i relaksatsiya stabilizirovannykh radikalov (ESR and Relaxation of Stabilized Radicals), Moscow: Khimiya, 1972, p. 25.
Wilson, R. and Kivelson, D., J. Chem. Phys., 1966, vol. 44, no. 1, p. 154.
Belford, G.G., Belford, R.L., and Burkhalter, J.F., J. Magn. Reson., 1973, vol. 11, no. 2, p. 251.
Yakovenko, A.V., Kolotilov, S.V., Cador, O., et al., Eur. J. Inorg. Chem., 2009, no. 16, p. 2354.
Utley, J.H.P. and Nielsen, M.F., Organic Electrochemistry, Lund, H. and Hammerich, O., N.Y., Eds., Marcel Dekker, Inc., 2001, p. 1227.
McCann, M., Cronin, J.F., Devereux, M., et al., Polyhedron, 1995, vol. 14, nos. 23−24, p. 3617.
Datta, D. and Chakravorty, A., Inorg. Chem., 1983, vol. 22, no. 7, p. 1085.
Santra, P.K., Das, D., Misra, T.K., et al., Polyhedron, 1999, vol. 18, no. 14, p. 1909.
McCreery, R.L., Chem. Rev., 2008, vol. 108, no. 7, p. 2646.
Khrizanforov, M.N., Arkhipova, D.M., Sheku-rov, R.P., et al., J. Solid State Electrochem., 2015, vol. 19, no. 9, p. 2883.
Fomina, I., Dobrokhotova, Zh., Aleksandrov, G., et al., Polyhedron, 2010, vol. 29, no. 7, p. 1734.
Zhang, C. and Jiao, N., Angew. Chem., Int. Ed., 2010, vol. 49, no. 35, p. 6174.
Allen, S.E., Walvoord, R.R., Padilla-Salinas, R., and Kozlowski, M.C., Chem. Rev., 2013, vol. 113, no. 8, p. 6234.
ACKNOWLEDGMENTS
The X-ray structure analysis, elemental analysis, and ESR spectroscopy of the obtained compounds and the magnetochemical measurements for compound II were performed at the User Facilities Center of IGIC RAS within the State Assignment on Fundamental Research to the Kurnakov Institute of General and Inorganic Chemistry (Russian Academy of Sciences).
This work was supported by the Russian Science Foundation, project no. 14-23-00176.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the 90th birthday of Academician I.I. Moiseev
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Nikolaevskii, S.A., Kiskin, M.A., Starikov, A.G. et al. Atmospheric Oxygen Influence on the Chemical Transformations of 4,5-Dimethyl-1,2-Phenylenediamine in the Reactions with Copper(II) Pivalate. Russ J Coord Chem 45, 273–287 (2019). https://doi.org/10.1134/S1070328419040067
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328419040067



