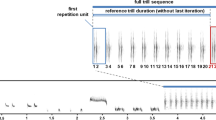
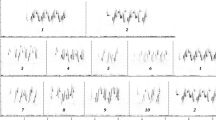
Abstract
Songbirds are known to use several different ways to signal aggression, and even closely-related species can differ in this respect. It is not clear why the variability exists among species. Here, we used playback experiments to compare the vocal behavior of three closely-related songbird species (Phylloscopus omeiensis, P. tephrocephalus and P. valentin) during simulated territorial intrusion. First of all, we analyzed spontaneous singing of each species and found that species differed quantitatively but not qualitatively. P. valentini sang with fewer song types and with more diverse song type sequencing patterns than both P. tephrocephalus and P. omeiensis. Species differed in how much they modified their spontaneous singing while responding to playback. P. valentini males modified singing the most as they increased song type switching rate and tended to sequence song types according to a fixed linear order while responding to playback. P. omeiensis increased song type switching rate, and P. tephrocephalus modified singing the least. We correlated singing in the two contexts (playback vs. spontaneous) both between and within each species. We found that species/males that tended to produce song types according to a fixed sequence and switch after every song type modified their singing less in aggressive contexts (e.g., P. tephrocephalus).




Similar content being viewed by others
REFERENCES
Akçay, K., Porsuk, Y.K., Avşar, A., Çabuk, K., and Bilgin, C.C., Song overlapping, noise, and territorial aggression in great tits, Behav. Ecol., 2020, vol. 31, no. 3, pp. 807–814.
Alström, P. and Olsson, U., The golden–spectacled warbler: a complex of sibling species from Sichuan Province, China, Ibis, 1999, vol. 141, pp. 545–568.
Alström, P. and Olsson, U., Golden-spectacled Warbler systematics, Ibis, 2000, vol. 142, pp. 495–500.
Alström, P., Olsson, U., and Lei, F., A review of the recent advances in the systematic of the avian superfamily Sylvioidea, Chin. Birds, 2013, vol. 4, pp. 99–131.
Baker, T., Wilson, D., and Mennill, D., Vocal signals predict attack during aggressive interactions in black-capped chickadees, Anim. Behav., 2012, vol. 84, pp. 965–974.
Bates, D., Maechler, M., Bolker, B., and Walker, S., Fitting linear mixed-effects models using lme4, J. Stat. Soft., 2015, vol. 67, pp. 1–48.
Bhattacharya, H., Cirillo, J., Subba, B.R., and Todt, D., Song performance rules in the oriental magpie robin (Copsychus salauris). Our Nat., 2007, vol. 5, pp. 1–13.
Briefer, E., Osiejuk, T., Rybak, F., and Aubin, T., Are bird song complexity and song sharing shaped by habitat structure? An information theory and statistical approach, J. Theor. Biol., 2010, vol. 262, pp. 151–164.
Burt, J.M. and Beecher, M.D., The social interaction role of song in song sparrow: implication for signal design, Comp. Cong. Behav. Rev., 2008, vol. 3, pp. 86–98.
Byers, E.B. and Kroodsma, E.D., Female mate choice and songbird repertoires, Anim. Behav., 2009, vol. 77, pp. 13–22.
Carlson, N.V., Healy, S.D., and Templeton, C.H., A comparative study of how British tits encode predator threat in their mobbing calls, Anim. Behav., 2017, vol. 125, pp. 77–92.
Catchpole, C.K. and Slater, P.J.B., Bird Song: Biological Themes and Variations, Cambridge: Cambridge Univ. Press, 2008.
Collins, S.A., Is female preference for male repertoires due to sensory bias?, Proc. R. Soc. B, 1999, vol. 266, pp. 2309–2314.
Collins, S.A., Vocal fighting and flirting: the functions of birdsong, in Nature’s Music: The Science of Birdsong, Marler, P. and Slabbekoorn, H., Eds., Amsterdam: Elsevier Academic Press, 2004, pp. 39–79.
Darolová, A., Krištofik, J., Knauer, F., and Hoi, H., Behavioural response of Eurasian Blackcaps to acoustically simulated conspecific and heterospecific male intruders, J. Ornithol., 2020, vol. 161, pp. 447–458.
Dobson, C.W. and Lemon, R.E., Markov sequences in songs of American thrushes, Behaviour, 1978, vol. 68, pp. 86–104.
Falls, J.B. and D’Agincourt, L.G., Why do meadowlarks switch song types?, Can. J. Zool., 1982, vol. 60, pp. 3400–3408.
Ficken, M.S. and Ficken, R.W., Comparative ethology of the chestnut-sided warbler, yellow warbler, and American redstart, Wilson Bull., 1965, vol. 77, pp. 363–375.
Flower, T.P., Gribble, M., and Ridley, A.R., Deception by flexible alarm mimicry in an African bird, Science, 2014, vol. 344, pp. 513–516.
Gentner, T., Mechanisms of temporal auditory pattern learning in song birds, Lang. Learn. Dev., 2007, vol. 3, pp. 1–22.
Gil, D. and Slater, P.J.B., Song organization and singing patterns of the willow warbler, Phylloscopus trochilus, Behaviour, 2000, vol. 137, pp. 759–782.
Goretskaia, M.I., Song structure variability in passerine birds: random variation or direct informative changes, Biol. Bull. (Moscow), 2013, vol. 40, pp. 748–759.
Grieves, L.A., Logue, D.M., and Quinn, J.S., Ready to fight: reliable predictors of attack in a cooperatively breeding, non-passerine bird, Ethology, 2015, vol. 121, pp. 1154–1165.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D., PAST—paleontological statistics, 2001. http://www.toyen.uio.no/~ohamm er/past.
Hesler, N., Mundry, R., and Dabelsteen, T., Does song repertoire size in common blackbirds play a role in an intra-sexual context?, J. Ornithol., 2011, vol. 152, pp. 591–601.
Hill, S.D., Brunton, D.H., Anderson, M., and Ji, W., Fighting talk: complex song elicits more aggressive responses in a vocally complex songbird, Ibis, 2018, vol. 160, pp. 257–268.
del Hoyo, J. and Collar, N.J., HBW and BirdLife International Checklist of the Birds of the World, Barcelona: Lynx Edicions, 2016.
Hurlbert, S.H., Pseudoreplication and the design of ecological field experiments, Ecol. Monogr., 1984, vol. 54, pp. 187–211.
Ivanitskii, V.V., Marova, I.M., and Malykh, I.M., Between order and chaos: contrasting syntax in the advertising song of dusky (Phylloscopus fuscatus) and Radde’s (Ph. schwarzi) warblers, J. Ornithol., 2012, vol. 153, pp. 337–346.
Ivanitskii, V.V., Marova, I.M., and Antipov, V.A., Sequential organization in the song of (Luscinia luscinia): clustering and sequential order of the song types, Bioacoustics, 2017, vol. 26, pp. 199–215.
Johansson, U.S., Alström, P., Olsson, U., Ericson, P.G., Sundberg, P., and Price, T.D., Build-up of the Himalayan avifauna through immigration: a biogeographical analysis of the Phylloscopus and Seicercus warblers, Evolution, 2007, vol. 61, pp. 324–333.
Kolesnikova, Y., Liu, M., Kang, Z., and Opaev, A., Song does not function as a signal of direct aggression in two leaf-warbler species, Ornithol. Sci., 2019, vol. 18, pp. 17–26.
Krebs, J.R., Habituation and song repertoires in the great tit, Behav. Ecol. Sociobiol., 1976, vol. 1, pp. 215–227.
Krebs, J.R., Song and territory in the great tit, in Evolutionary Ecology, Stonehouse, B. and Perrins, C., Eds., New York: Macmillan, 1977, pp. 47–62.
Krebs, J.R., Ashcroft, R., and Orsdol, K.V., Song matching in the great tit Parus major L., Anim. Behav., 1981, vol. 29, pp. 918–923.
Kroodsma, D.E., Continuity and versatility in bird song: support for the monotony-threshold hypothesis, Nature, 1978, vol. 274, pp. 681–683.
Kroodsma, D.E., Suggested experimental designs for song playbacks, Anim. Behav., 1989, vol. 37, pp. 600–609.
Kroodsma, D.E. and Verner, J., Complex singing behaviors among Cistothorus wrens, Auk, 1978, vol. 95, pp. 703–716.
Kroodsma, D.E., Byers, B.E. Goodale, E., Johnson, S., and Lui, W.-C., Pseudoreplication in playback experiments, revisited a decade later, Anim. Behav., 2001, vol. 61, pp. 1029–1033.
Kuznetsova, A., Brockhoff, P.B., and Christensen, R.H.B., lmerTest package: tests in linear mixed effects models, J. Stat. Soft., 2017, vol. 82, pp. 1–26.
Leitão, A., ten Cate, C., and Riebel, K., Within-song complexity in a songbird is meaningful to both male and female receivers, Anim. Behav., 2006, vol. 71, pp. 1289–1296.
Lemon, R.E., Cotter, R., MacNally, R.C., and Monette, S., Song repertoires and song sharing by American redstarts, Condor, 1985, vol. 87, pp. 457–470.
Lemon, R.E., Dobson, C.W., and Clifton, P.G., Songs of American redstarts (Setophaga ruticilla): sequencing rules and their relationships to repertoire size, Ethology, 1993, vol. 93, pp. 198–210.
Linhart, P., Slabbekoorn, H., and Fuchs, R., The communicative significance of song frequency and song length in territorial chiffchaffs, Behav. Ecol., 2012, vol. 23, pp. 1338–1347.
McGregor, P.K., Playback experiments: design and analysis, Acta Ethol., 2000, vol. 3, pp. 3–8.
McGregor, P.K., Dablesteen, T., Shepherd, M., and Pedersen, S.B., The signal value of matched singing in great tits: evidence from interactive playback experiments, Anim. Behav., 1992, vol. 43, pp. 987–998.
Marler, P. and Slabbekoorn, H., Nature’s Music, The Science of Birdsong, San Diego: Academic, 2004.
Martens, J., A preliminary review of the leaf warbler genera Phylloscopus and Seicercus, Br. Ornithol. Club Occas. Publs., 2010, vol. 5, pp. 41–116.
Martens, J., Eck, S., Päckert, M., and Sun, Y.-H., The golden-spectacled warbler Seicercus burkii: a species swarm (Aves: Passeriformes: Sylviidae). Part 1, Zool. Abh. (Dresden), 1999, vol. 50, pp. 282–327.
Martens, J., Eck, S., Päckert, M., and Sun, Y.-H., Methods of systematic and taxonomic research on passerine birds: the timely example of the Seicercus burkii complex (Sylviidae). Part 2, Bonner Zool. Beitr., 2003, vol. 51, pp. 109–118.
Mennill, D.J. and Ratcliffe, L.M., Overlapping and matching in the song contests of black-capped chickadees, Anim. Behav., 2004, vol. 67, pp. 441–450.
Molles, L.E., Singing complexity of the banded wren Thryothorus pleurostictus: do switching and song-type diversity send different messages?, Auk, 2006, vol. 123, pp. 991–1003.
Naguib, M., Effects of song overlapping and alternating on nocturnally singing nightingales, Anim. Behav., 1999, vol. 58, pp. 1061–1067.
Nowicki, S., Searcy, W.A., and Hughes, M., The territory defense function of song in song sparrows: a test with the speaker occupation design, Behaviour, 1998, vol. 135, pp. 615–628.
Okanoya, K., Finite–state song syntax in Bengalese finches: sensorimotor evidence, developmental processes, and formal procedures for syntax extraction, in Birdsong, Speech, and Language. Exploring the Evolution of Mind and Brain, Bolhuis, J.J. and Everaert, M., Eds., London: The MIT Press, 2013, pp. 229–242.
Opaev, A., Relationships between repertoire size and organization of song bouts in the grey-crowned warbler (Seicercus tephrocephalus), J. Ornithol., 2016, vol. 157, pp. 949–960.
Opaev, A.S. and Kolesnikova, Y.A., The role of song rate and song bout’s complexity in the territorial behavior of Radde’s warbler (Phylloscopus schwarzi), Zool. Zh., 2019a, vol. 98, pp. 319–331.
Opaev, A. and Kolesnikova, Y., Lack of habitat segregation and no interspecific territoriality in three syntopic cryptic species of the golden-spectacled warblers Phylloscopus (Seicercus) burkii complex, J. Avian. Biol., 2019b, vol. 50, pp. 1–9.
Opaev, A., Kolesnikova, Y., Liu, M., and Kang, Z., Singing of Claudia’s leaf-warbler (Phylloscopus claudiae) in aggressive contexts: role of song rate, song type diversity and song type transitional pattern, J. Ornithol., 2019, vol. 160, pp. 297–304.
Päckert, M., Martens, J., Sun, Y.-H., and Veith, M., The radiation of the Seicercus burkii complex and its congeners (Aves: Sylviidae): molecular genetics and bioacoustics, Org. Div. Evol., 2004, vol. 4, pp. 341–364.
Päckert, M., Sun, Y.-H., Peterson, T., Holt, P., Strutzenberger, P., and Martens, J., Integrative taxonomy of Seicercus spectacled warblers for mapping species’ distribution, Atlas Verbr. Palaearkt. Vogel, 2016, vol. 22, pp. 1–7.
Pitt, S.M.G., Why sing so many songs? Testing the function of song type repertoires in rock wrens using playback experiments and behavioral observations, Master of Science Thesis, University of Northern Colorado, 2018.
R Core Team, R: A Language and Environment for Statistical Computing, Vienna: R Found. Stat. Comp., 2020. https://www.R-project.org.
Randler, C., A possible phylogenetically conserved urgency response of great tits (Parus major) towards allopatric mobbing calls, Behav. Ecol. Sociobiol., 2012, vol. 66, pp. 675–681.
Riebel, K. and Slater, P.J.B., Song type switching in the chaffinch, Fringilla coelebs: timing or counting?, Anim. Behav., 1999, vol. 57, pp. 655–661.
Sasahara, K., Cody, M.L., Cohen, D., and Taylor, C., Structural design principles of complex bird songs: a network-based approach, PLoS One, 2012, vol. 7, p. e44446.
Schank, J.C. and Koehnle, T.J., Pseudoreplication is a pseudoproblem, J. Comp. Psychol., 2009, vol. 123, pp. 421–433.
Scharff, C. and Nottebohm, F., A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implication for vocal learning, J. Neurosci., 1991, vol. 11, pp. 2896–2913.
Scordato, E.S.C., Geographic variation in male territory defense strategy in an avian ring species, Anim. Behav., 2017, vol. 126, pp. 153–162.
Searcy, W.A. and Beecher, M.D., Song as an aggressive signal in songbirds, Anim. Behav., 2009, vol. 78, pp. 1281–1292.
Searcy, W.A. and Yasukawa, K., Use of the song repertoire in intersexual and intrasexual context by male red-winged blackbirds, Behav. Ecol. Sociobiol., 1990, vol. 27, pp. 123–128.
Searcy, W.A., Nowicki, S., and Hogan, C., Song type variants and aggressive context, Behav. Ecol. Sociobiol., 2000, vol. 48, pp. 358–363.
Searcy, W.A., Anderson, R.C., and Nowicki, S., Bird song as a signal of aggressive intent, Behav. Ecol. Sociobiol., 2006, vol. 60, pp. 234–241.
Spector, D.A., The singing behaviour of yellow warblers, Behaviour, 1991, vol. 117, pp. 29–52.
Spedicato, G.A., Kang, T.S., Yalamanchi, S.B., Thoralf, M., Yadav, D., Castillo, N.C., and Jain, V., Easy handling discrete time Markov chains, 2017. https://cran.r-project.org/web/packages/markovchain.
Suzuki, T.N., Semantic communication in birds: evidence from field research over the past two decades, Ecol. Res., 2016, vol. 31, pp. 307–319.
Szymkowiak, J. and Kuczyński, L., Song rate as a signal of male aggressiveness during territorial contests in the wood warbler, J. Avian Biol., 2017, vol. 48, pp. 275–283.
Todt, D. and Hultsch, H., How songbirds deal with large amounts of serial information: retrieval rules suggest a hierarchical song memory, Biol. Cybern., 1998, vol. 79, pp. 487–500.
Todt, D. and Naguib, M., Vocal interactions in birds: the use of song as a model in communication, Adv. Study Behav., 2000, vol. 29, pp. 247–296.
Vehrencamp, S.L. Handicap, index, and conventional signal elements of bird song, in Animal Signals: Signalling and Signal Design in Animal Communication, Espmark, Y., Amundsen, T., Rosenqvist, G., Eds., Trondheim: Tapir Publishers, 2000, pp. 277–300.
Vehrencamp, S.L., Hall, M.L., Bohman, E.R., Depeine, C.D., and Dalziel, A.H., Song matching, overlapping, and switching in the banded wren: the sender’s perspective, Behav. Ecol., 2007, vol. 18, pp. 849–859.
Weiss, M., Hultsch, H., Adam, I., Scharff, C., and Kipper, S., The use of network analysis to study complex animal communication systems: a study on nightingale song, Proc. R. Soc. B, 2014, vol. 281, p. 20140460.
Woolley, S.M.N. and Rubel, E.W., Bengalese finches Lonchura striata domestica depend upon auditory feedback for the maintenance of adult song, J. Neurosci., 1997, vol. 17, pp. 6380–6390.
ACKNOWLEDGMENTS
The authors thank Shurong Tian, Meishi Liu and Zujie Kang for their support during field study.
Funding
The study was supported by the Russian Science Foundation (grant no. 20-14-00058-P).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All applicable institutional and/or national guidelines for the care and use of animals were followed. As the work was observational, authors did not need to provide ethical clearance.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Kolesnikova, Y.A., Opaev, A.S. Comparative Study of Aggressive Signaling in Three Closely-Related Warbler Species. Biol Bull Russ Acad Sci 50 (Suppl 3), S415–S427 (2023). https://doi.org/10.1134/S1062359023602495
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359023602495