Abstract
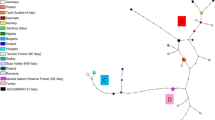
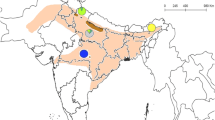
The red deer (Cervus elaphus) has been translocated across Europe, including Poland in order to improve the quality of local populations of the animal. The aim of the study was to assess the result of the translocations by microsatellite DNA analysis based determination of phylogenetic differences between red deer populations from seven regions of Poland, i.e. Lubelskie, Warmińsko-Mazurskie, Pomorskie, Zachodniopomorskie, Opolskie, Śląskie, and Wielkopolskie Provinces. Loci BMC1009, IDVGA55, INRA121, NVHRT48, CSSM41, and BM757 were analysed. All analyses were performed with the consent of the Local Ethics Committee 0071, Resolution No. 13/2014. The mean value of Ho in the red deer populations was at a level of 0.457, He 0.613, and the PIC value in all red deer groups was higher than 5.000, which indicates a large genetic diversity in this species in Poland. The value of the FST coefficient (0.17) was similar to the mean value noted in Cervus elaphus in Europe (0.166). However, the FIS and FIT coefficients were relatively high (0.259 and 0.334, respectively), which may imply the existence of internal structures in individual subpopulations. Additionally, the bottleneck effect could not be excluded due to the negative FIS and FIT values in locus BMC1009 (–0.260 and –0.071, respectively) and the excess of He relative to Heq.

Similar content being viewed by others
REFERENCES
Hartl, B.G., Zachos, F., and Nadlinger, K., Genetic diversity in European red deer (Cervus elaphus L.) antropogenetic influences on natural populations, Biologies, 2003, vol. 326, pp. 37–42.
Niedziałkowska, M., Jędrzejewska, B., Honnen, A.C., et al., Molecular biogeography of red deer Cervus elaphus from Eastern Europe: insights from mitochondrial DNA sequences, Acta Theriol., 2011, vol. 56, no. 1, pp. 1–12.
Niedziałkowska, M., Jędrzejewska, B., Wójcik, J.M., and Goodman, S.J., Genetic structure of red deer population in Northeastern Poland in relation to the history of human interventions, J. Wildlife Manage., 2012a, vol. 76, pp. 1264–1276.
Niedziałkowska, M., Fontaine, M.C., and Jędrzejewska, B., Factors shaping red deer gene flow in semi-natural landscapes of Central Europe, Can. J. Zool., 2012, vol. 90, no. 2, 150–162.
Bobek, B., Morow, K., Perzanowski, K., and Kossobudzka, M., The Red Deer—Its Ecology and Managemen, Warsaw, Świat, 1992.
Borowski, Z., Krysiuk, K., and Pudełko, M., Is the resettlement of animals reasonable in terms of population management? On the example of precious red deer (Cervus elaphus) and fallow deer (Dama dama), Stud. Mater.CEPL Rogów, 2013, vol. 36, no. 3, pp. 79–87.
Dzięgielewski, S., Red Deer, Warsaw: PWRiL, 1970.
Tajchman, K. and Drozd, L., Management of hunting animals population as breeding work: 2. Hunting and breeding work on red deer (Cervus elaphus) and elk (Alces alces) populations, Ann. Warsaw Univ. of Life Sci.—SGGW,Anim. Sci., 2018, vol. 57, no. 3, pp. 299—309.
Drozd, L., Pięta, M., Karpiński, M., and Piwniuk, J., The quality of deer in the macroregion of central-eastern Poland, Sylwan, 2000, vol. 3, pp. 87–92.
Panek, M. and Budny, M., The Situation of Game Animals in Poland with Particular Regard to Partridges (Based on Monitoring), Research Station of the PZŁ Czempiń, 2015.
Zachos, F.E., Althoff, C., Steynitz, Yv., et al., Genetic analysis of an isolated red deer (Cervus elaphus) population showing signs of inbreeding depression, Eur. J. Wildlife Res., 2007, vol. 53, pp. 61–67.
Crnokrak, P. and Roff, D.A., Inbreeding depression in the wild, Heredity, 1999, vol. 83, pp. 260–270.
Bonnet, A., Thévenon, S., Maudet, F., and Maillard, J.C., Efficiency of semi-automated fluorescent multiplex PCRs with 11 microsatellite markers for genetic studies of deer population, International Society for Animal Genetics, Anim. Genet., 2002, vol. 33, pp. 343–350.
Galan, M., Cosson, J.F., Aulagnier, S., et al., Cross-amplification tests of ungulate primers in roe deer (Capreolus capreolus) to develop a multiplex panel of 12 microsatellite loci, Mol. Ecol. Notes, 2003, vol. 3, pp. 142—146.
Piry, S.G., Luikart, G., and Cornuet, J.M., Bottleneck: a computer program for detecting recent reductions in the effective population size using allele frequency data, J. Hered., 1999, vol. 90, pp. 502–503.
Cornuet, J.M. and Luikart, G., Description and evaluation of two tests for detecting recent bottlenecks, Genetics, 1996, vol. 144, pp. 2001–2014.
The Princeton Guide to Ecology, Levin, S.A., Ed., Princeton University Press, 2009.
Borowski, Z.S., Świsłocka, M., Matosiuk, M., et al., Purifying selection, density blocking and unnoticed mitochondrial DNA diversity in the red deer (Cervus elaphus), PLoS One, 2016, vol. 11, no. 9, pp. 1–17.
Thévenon, S., Thuy, L.T., Ly, L.V., et al., Microsatellite analysis of genetic diversity of the Vietnamese sika deer (Cervus nippon pseudaxis), J. Hered., 2004, vol. 95, no. 1, pp. 11–18.
Zachos, F.E., Frantz, A.C., Kuehn, R., et al., Genetic structure and effective population size in European red deer (Cervus elaphus) at a continental scale: insights from microsatellite DNA, J. Hered., 2016, vol. 107, no. 4, pp. 318–326.
Pakeman, R.J., Consistency of plant species and trait responses to grazing along a productivity gradient: a multi-site analysis, J. Ecol., 2004, vol. 92, pp. 893–905.
Jarnemo, A., Seasonal migration of male red deer (Cervus elaphus) in southern Sweden and consequences for management, Eur. J. Wildlife Res., 2008, vol. 54, no. 2, pp. 327–333.
Borowik, T., Cornulier, T., and Jędrzejewska, B., Environmental factors shaping ungulate abundances in Poland, Acta Theriol., 2013, vol. 58, pp. 403–413.
Kuehn, R., Schroeder, W., Pirchner, F., and Rottmann, O., Genetic diversity, gene flow and drift in Bavarian red deer population (Cervus elaphus), Conserv. Genet., 2003, vol. 4, pp. 157–166.
Feulner, P.G.D., Bielfeldt, W., Zachos, F.E., et al., Mitochondrial DNA and microsatellite analyses of the genetic status of the presumed subspecies Cervus elaphus montanus (Carpathian red deer), Heredity, 2004, vol. 93, pp. 299–306.
Martínez, J.G., Carranza, J., Fernández-García, J.L., and Sánchez-Prieto, C.B., Genetic variation of red deer populations under hunting exploitation in southwestern Spain, J. Wildlife Manage., 2002, vol. 66, pp. 1273–1282.
Lorenzini, R., Mattioli, S., and Fico, R., Allozyme variation in native red deer (Cervus elaphus) of Mesola Wood, northern Italy: implications for conservation, Acta Theriol., 1998, vol. 5, pp. 63–74.
Hundertmark, K.J. and Van Daele, L.J., Founder effect and bottleneck signatures in an introduced, insular population of elk, Conserv. Genet, 2010, vol. 11, pp. 139–148.
Höglund, J., Cortazar-Chinarro, M., Jarnemo, A., and Thulin, C.G., Genetic variation and structure in Scandinavian red deer (Cervus elaphus): influence of ancestry, past hunting and restoration management, Biol. J. Linn. Soc., 2013, vol. 109, pp. 43–53.
Lavsund, S., Kronhjortens, Cervus elaphus L., utbredning i Sverige1900–1973, Stockholm: Skogshögskolan, 1975, no. 18, р. 49.
Hedrick, P.W., Perspective: highly variable loci and their interpretation in evolution and conservation, Evolution, 1999, vol. 53, pp. 313–318.
Hedrick, P.W., Conservation genetics: where are we now?, Trends Ecol. Evol., 2001, vol. 16, pp. 629–636.
Janiszewski, P. and Szczepański, W., Characterization of masses of deer stags, deer does and deer fawns (Cervus elaphus L.) obtained during autumn and winter, Sylwan, 2004, vol. 1, pp. 33–38.
Janiszewski, P. and Kolasa, S., Biometric characteristics of roebucks (Capreolus capreolus) from Tabórz Forests, Poland, Balt. For., 2007, vol.13, no. 2, pp. 215–220.
Krupka, J., Dziedzic, R., and Drozd, L., Quality characteristics of red deer stags obtained in the macroregion of central eastern Poland, Ann. Univ.Mariae Curie-Skłodowska, 1986, vol. 4, pp. 7–14.
Szczepański, W., Janiszewski, P., and Kolasa, S., Zoometric studies on deer fawns (Cervus elaphus L.) from the breeding area “Taborskie Forest,” Sylwan, 2006, vol. 5, pp. 16–23.
Meredith, E.P., Rodzen, J.A., Banks, J.D., et al., Microsatellite analysis of three subspecies of elk (Cervus elaphus) in California, J. Mammal., 2007, vol. 88, no. 3, pp. 801–808.
Linnell, J.D.C. and Zachos, F.E., Status and distribution patterns of European ungulates: genetics, population history and conservation, in Ungulate Management in Europe: Problems and Practices, Putman, R., Apollonio, M., and Andersen, R., Eds., Cambridge: Cambridge University Press, 2011, pp.12–53.
Niethammer, G., Die Einbürgerung von Säugetieren und Vögeln in Europa, Hamburg: Paul Parey, 1963.
Perzanowska, J., Makomaska-Juchiewicz, M., Cierlik, G., et al., Ecological Corridors in Małopolska, Institute of Environmental Sciences, Jagiellonian University, Institute of Nature Conservation PAN, 2005.
Tajchman, K., Drozd, L., Karpiński, M., et al., Population genetic structure of wild boars in Poland, Russ. J. Genet., 2018, vol. 54, no. 5, pp. 548–553.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Tajchman, K., Sawicka-Zugaj, W., Greguła-Kania, M. et al. Effect of Translocations on the Genetic Structure in Populations of the Red Deer (Cervus elaphus) in Poland. Russ J Genet 55, 1506–1513 (2019). https://doi.org/10.1134/S102279541912010X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102279541912010X