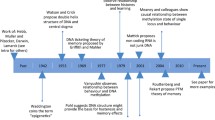
Abstract
Behavioral genetics or “Neurogenetics,” is based on the evolutionary ideas of T. Dobzhansky on brain development and behavior. It continues with the “experimental genetics of higher nervous activity” of I. Pavlov and uses a comparative approach in the study of heredity and variation in behavioral manifestations, from Protozoa to humans. The study of the classical Pavlovian conditioned reflex in mutant Drosophila helped to identify the main types of memory and their evolutionary conservatism. Long-term memory defects are caused by mutations of the same genes as in mental retardation in humans, when signaling cascades intersecting with the cAMP-dependent pathway are damaged. The cascade of actin remodeling is also among these. The key enzyme, LIM-kinase 1, controls cognitive manifestations of the “genomic disease” Williams deletion syndrome. Its study resulted in the recognition of neuroepigenetics as an interface between the genome and environmental influences. Epigenetic factors of “variability”—DNA methylation, histone acetylation, and miRNA regulation—do not change the structure of the gene but its manifestations. Certain miRNAs have already been considered to be both biomarkers for neurodegenerative diseases and factors of the transgenerational transmission of the behaviorial properties of ancestors who experienced stress from adverse environmental influences.
Similar content being viewed by others
References
Fuller, J.R. and Thompson, W.R., Behavior Genetics, New York: Wiley, 1960.
Galton, F., Hereditary Genius: Its Laws and Consequences, London: Macmillan, 1869.
Korochkin, L.I. and Mikhailov, A.T., Vvedenie v neirogenetiku (Introduction to Neurogenetics), Moscow: Nauka, 2000.
Fuller, J.L., Comparative studies in behavioral genetics, Acta Genet. Stat. Med., 1957, vol. 7, no. 2, pp. 403–407.
Fuller, J.L., Physiological and population aspects of behavior genetics, Am. Zool., 1964, vol. 4, pp. 101–109.
Thompson, W.R., The inheritance and development of intelligence, Res. Publ. Assoc. Res. Nerv. Ment. Dis., 1954, vol. 33, pp. 209–231.
Scott, J.P., Social genetics, Behav. Genet., 1977, vol. 7, pp. 327–346.
Niemi, R.R. and Thompson, W.R., Pavlovian excitation, internal inhibition, and their interaction with free operant avoidance as a function of age in rats, Dev. Psychobiol., 1980, vol. 13, pp. 61–76.
Ehrman, L, and Parsons, P A., Behavior, Genetics and Evolution, New York: McGraw Hill, 1981.
Mazing, R.A., Inheritance of oviposition selectivity in Drosophila melanogaster, Dokl. Akad. Nauk SSSR, 1946, vol. 51, no. 7, pp. 534–546.
Mazing, R.A., Variation and heredity of photoreactions in flies Drosophila melanogaster, Zh. Obshch. Biol., 1943, vol. 4, no. 4, pp. 209–231.
Benzer, S., From the gene to behavior, JAMA, 1971, vol. 218, no. 7, pp. 1015–1022.
Pavlov, I.P., Twenty Years Experience in Objective Study of Higher Nervous Activity (Behavior) of Animals, in Polnoe sobranie sochinenii AN SSSR 1951–1954 (Complete Set of Works of Academy of Sciences of the Soviet Union 1951–1954), Moscow: Nauka, vol. 3, book 2, 2nd ed., 1973.
Dubnau, J., Chiang, A.S., and Tully, T., Neural substrates of memory: from synapse to system, J. Neurobiol., 2003, vol. 54, no. 1, pp. 238–253.
Baranov, M.S., Genome paths: a way to personalized and predictive medicine, Acta Nat., 2009, vol. 1, no. 3, pp. 70–80.
Pavlov, I., Conditioned Reflexes, New York: Oxford Univ. Press, 1927.
Benzer, S., The fine structure of the gene, Sci. Am., 1962, vol. 206, pp. 70–84.
Hotta, Y. and Benzer, S., Mapping of behaviour in Drosophila mosaics, Nature, 1972, vol. 240, no. 5383, pp. 527–535.
Benzer, S., Genetic dissection of behavior, Sci. Am., 1973, vol. 229, no. 6, pp. 24–37.
Benzer, S., From gene to behavior, Aktual’nye problemy genetiki povedeniya (Current Issues in Behavior Genetics), Fedorov, V.K. and Ponomarenko, V.V., Eds., Leningrad: Nauka, 1975.
Dudai, Y., Jan, Y.N., Byers, D., et al., dunce, a mutant of Drosophila deficient in learning, Proc. Natl. Acad. Sci. U.S.A., 1976, vol. 73, no. 5, pp. 1684–1688.
Davis, R.L. and Davidson, N., Isolation of the Drosophila melanogaster dunce chromosomal region and recombinational mapping of dunce sequences with restriction site polymorphisms as genetic markers, Mol. Cell Biol., 1984, vol. 4, no. 2, pp. 358–367.
Margulies, C., Tully, T., and Dubnau, J., Deconstructing memory in Drosophila, Curr. Biol., 2005, vol. 15, no. 17, pp. 700–713.
Chen, C.C., Wu, J.K., Lin, H.W., et al., Visualizing long-term memory formation in two neurons of the Drosophila brain, Science, 2012, vol. 335, no. 6069, pp. 678–685.
Zhuravlev, A.V., Nikitina, E.A., and Savvateeva-Popova, E.V., Learning and memory in Drosophila: physiological and genetic basis, Usp. Fiziol. Nauk, 2015, vol. 46, no. 1, pp. 75–90.
Janus, C. and Dubnau, J., Modeling behavior: the quest to link mechanisms to function, Genes. Brain Behav., 2003, vol. 2, no. 1, pp. 56–61.
Dubnau, J., Neurogenetic dissection of conditioned behavior: evolution by analogy or homology?, J. Neurogenet., 2003, vol. 17, no. 4, pp. 295–326.
Lobashev, M.E., On parallel analogous and homologous series of development of basic properties of higher nervous activity in animal phylogeny, in Materialy 2-go nauchogo soveshchaniya po evolyutsionnoi fiziologii, posvyashchennogo pamyati akademika L.A. Orbeli (Proceedings of the 2nd Scientific Conference on Evolutionary Physiology, dedicated to the memory of Academician L.A. Orbely), Moscow, 1960, pp. 16–23.
Lobashev, M.E., Signaling heredity, in Issledovaniya po genetike (Research in Genetics), Leningrad: Leningrad Gos. Univ., 1961, no. 1, pp. 3–11.
Germana, J., A transactional analysis of biobehavioral systems, Integr. Physiol. Behav. Sci., 1996, vol. 31, no. 3, pp. 210–218.
Karmiloff-Smith, A., Williams syndrome, Curr. Biol., 2007, vol. 17, no. 24, pp. 1035–1036.
Poulsen, P., Esteller, M., Vaag, A., and Fraga, M.F., The epigenetic basis of twin discordance in age-related diseases, Pediatr. Res., 2007, vol. 61, pp. 38–42.
Jirtle, R.L., Environmental epigenomics and disease susceptibility, Nat. Rev. Genet., 2007, vol. 8, pp. 253–262.
Skinner, M.K., Anway, M.D., Savenkova, M.I., et al., Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior, PLoS One, 2008, vol. 3, no. 11. e3745
Crews, D., Gillette, R., Scarpino, S.V., et al., Epigenetic transgenerational inheritance of altered stress responses, Proc. Natl. Acad. Sci. U.S.A., 2012, vol. 109, no. 23, pp. 9143–9148.
Dias, B.G. and Ressler, K.J., Parental olfactory experience influences behavior and neural structure in subsequent generations, Nat. Neurosci., 2014, vol. 17, no. 1, pp. 89–96.
Fang, L., Wuptra, K., Chen, D., et al., Environmentalstress-induced chromatin regulation and its heritability, J. Carcinog. Mutagen., 2014, vol. 5, no. 1, p. 22058.
Heisnberg, M., Pavlov’s dog and Benzer’s fly: the genetics of higher nervous activity, Neurosci. Behav. Physiol., 1997, vol. 27, no. 5, pp. 632–634.
Tully, T., Pavlov’s dogs, Curr. Biol., 2003, vol. 13, no. 4, pp. 117–119.
Barditch, J., Gossweiler, S., McNeil, J., et al., The staufen/pumilio pathway is involved in Drosophila long-term memory, Curr. Biol., 2003, vol. 13, no. 4, pp. 286–296.
Bolduc, F.V. and Tully, T., Fruit flies and intellectual disability, Fly (Austin), 2009, vol. 3, no. 1, pp. 91–104.
Landry, C.D., Kandel, E.R., and Rajasethupathy, P., New mechanisms in memory storage: piRNAs and epigenetics, Trends Neurosci., 2013, vol. 36, no. 9, pp. 535–542.
Vogel-Ciernia, A. and Wood, M.A., Neuron-specific chromatin remodeling: a missing link in epigenetic mechanisms underlying synaptic plasticity, memory, and intellectual disability disorders, Neuropharmacology, 2014, vol. 80, pp. 18–27.
Jarome, T.J. and Lubin, F.D., Epigenetic mechanisms of memory formation and reconsolidation, Neurobiol. Learn. Mem., 2014. pii: S1074-7427(14)00141-5
Contestabile, A., Sintoni, S., and Monti, B., Histone deacetylase (HDAC) inhibitors as potential drugs to target memory and adult hippocampal neurogenesis, Curr. Psychopharmacol., 2012, vol. 1. pp. 14–28. doi 10.2174/2211556011201010014.
Savvateeva-Popova, E.V., Popov, A.V., Nikitina, E.A., et al., Pathogenic chaperone-like RNA induces congophilic aggregates and facilitates neurodegeneration in Drosophila, Cell Stress Chaperones, 2007, vol. 12, no. 1, pp. 9–19.
Savvateeva-Popova, E., Popov, A., Grossman, A., et al., Non-coding RNA as a trigger of neuropathologic disorder phenotypes in transgenic Drosophila, J. Neuronal. Transm., 2008, vol. 115, no. 12, pp. 1629–1642.
Savvateeva-Popova, E.V., Medvedeva, Popov, A.V., et al., Role of non-coding RNAs in neurodegeneration and stress response in Drosophila, J. Biotechnol., 2008, vol. 3, no. 8, pp. 1010–1021.
Medvedeva, A.V., Zhuravlev, A.V., and Savvateeva-Popova, E.V., LIMK1, the key enzyme of actin remodeling bridges spatial organization of nucleus and neural transmission: from heterochromatin via non-coding RNAs to complex behavior, in Horizons in Neuroscience Research, New York, 2010, vol. 1, ch. 4, pp. 161–193.
Rumyantsev, A.M., Zakharov, G.A., Zhuravlev, A.V., et al., Expression of the Drosophila melanogaster Limk1 gene 3′-UTRs mRNA in yeast Saccharomyces cerevisiae, Russ. J. Genet., 2014, vol. 50, no. 6, pp. 569–578.
Lushnikov, S.G., Dmitriev, A.V., Fedoseev, A.I., et al., Low-frequency dynamics of DNA in Brillouin light scattering spectra, JETP Lett., 2013, vol. 98, no. 11, pp. 830–836.
Brázda, V., Laister, R.C., Jagelskrá, E.B., and Arrowsmith, C., Cruciform structures are a common DNA feature important for regulating biological processes, BMC Mol. Biol., 2011, vol. 12, p. 33.
Brázda, V., Háronríková, L., Liao, J.C., and Fojta, M., DNA and RNA quadruplex-binding proteins, Int. J. Mol. Sci., 2014, vol. 15, no. 10, pp. 17493–17517.
Hirsch, E.C., How to judge animal models of Parkinson’s disease in terms of neuroprotection, J. Neural. Transm., 2006, suppl., vol. 70, pp. 255–260.
Broadstock, M., Ballard, C., and Corbett, A., Latest treatment options for Alzheimer’s disease, Parkinson’s disease dementia and dementia with Lewy bodies, Expert Opin. Pharmacother., 2014, vol. 15, no. 13, pp. 1797–1810. doi 10.1517/14656566.2014.936848. Epub 2014 Jul 3.
Nikitina, E.A., Medvedeva, A.V., Zakharov, G.A., and Savvateeva-Popova, E.V., Williams syndrome as a model for elucidation of the pathway genes—the brain—cognitive functions: genetics and epigenetics, Acta Nat., 2014, vol. 6,no. 1, pp. 9–22.
Zucchi, F.C., Yao, Y., and Metz, G.A., The secret language of destiny: stress imprinting and transgerational origins of disease, Front. Genet., 2012. doi 10.3389/fgene.2012.00096.eCollection 2012
Bernstein, B.W. and Bamburg, J.R., ADF/cofilin: a functional node in cell biology, Trends Cell Biol., 2010, vol. 20, no. 4, pp. 187–195.
Walsh, K.P., Minamide, L.S., Kane, S.J., et al., Amyloid- and proinflammatory cytokines utilize a prion protein-dependent pathway to activate NADPH oxidase and induce cofilin-actin rods in hippocampal neurons, PLoS One, 2014, vol. 9, no. 4. e95995
Hughes, V., Sperm RNA carries marks of trauma, Nature, 2014, vol. 508, no. 7496, pp. 296–297.
Hughes, V., Epigenetics: the sins of the father, Nature, 2014, vol. 507, no. 7490, pp. 22–24.
Kuzin, B.A., Nikitina, E.A., Cherezov, R.O., et al., Combination of hypomorphic mutations of the Drosophila homologues of aryl hydrocarbon receptor and nucleosome assembly protein family genes disrupts morphogenesis, memory and detoxification, PLoS One, 2014, vol. 9, no. 4. e94975
Zheleznyakova, G.Y., Voisin, S., Kiselev, A.V., et al., Genome-wide analysis shows association of epigenetic changes in regulators of Rab and Rho GTPases with spinal muscular atrophy severity, Eur. J. Hum. Genet., 2013, vol. 21, no. 9, pp. 988–993.
Johnson, R., Noble, W., Tartaglia, G.G., and Buckley, N.J., Neurodegeneration as an RNA disorder, Prog. Neurobiol., 2012, vol. 99, pp. 293–315.
Maciotta, S., Meregalli, M., and Torrente, Y., The involvement of microRNAs in neurodegenerative diseases, Front Cell Neurosci., 2013, vol. 7, p. 265.
Gehrke, S., Imai, Y., Sokol, N., and Lu, B., Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression, Nature, 2010, vol. 466, pp. 637–641.
Venderova, K., Kabbach, G., Abdel-Messih, E., et al., Leucine-rich repeat kinase 2 interacts with Parkin, DJ-1 and PINK-1 in a Drosophila melanogaster model of Parkinson’s disease, Hum. Mol. Genet., 2009, vol. 18, no. 22, pp. 4390–4404.
Rogacheva, O.N., Shchegolev, B.F., Stefanov, V.E., et al., Initiation of the 3′: 5′-AMP induced protein kinase A Ia regulatory subunit conformational transition: 1. A202 and A326 are critical residues, Biochemistry (Moscow), 2012, vol. 77, no. 5, pp. 456–464.
Wu, Y., Chen, C., Mercer, A., and Sokol, N., Let-7-complex microRNAs regulate the temporal identity of Drosophila mushroom body neurons via chinmo, Dev. Cell, 2012, vol. 23, no. 1, pp. 202–209.
Kucherenko, M.M., Barth, J., Fiala, A., and Shcherbata, H.R., Steroid-induced microRNA let-7 acts as a spatio-temporal code for neuronal cell fate in the developing Drosophila brain, EMBO J., 2012, vol. 31, no. 24, pp. 4511–4523.
Redt-Clouet, C., Trannoy, S., Boulanger, A., et al., Mushroom body neuronal remodelling is necessary for short-term but not for long-term courtship memory in Drosophila, Eur. J. Neurosci., 2012, vol. 35, no. 11, pp. 1684–1691.
Sokol, N.S., Xu, P., Jan, Y.N., and Ambros, V., Drosophila let-7 microRNA is required for remodeling of the neuromusculature during metamorphosis, Genes Dev., 2008, vol. 22, no. 12, pp. 1591–1596.
Chawla, G. and Sokol, N.S., Hormonal activation of let-7-C microRNAs via EcR is required for adult Drosophila melanogaster morphology and function, Development, 2012, vol. 139, no. 10, pp. 1788–1797.
Zovoilis, A., Agbemenyah, H.Y., Agis-Balboa, R.C., et al., microRNA-34c is a novel target to treat dementias, EMBO J., 2011, vol. 30, pp. 4299–4308.
Bader, A.G., miR-34a microRNA replacement therapy is headed to the clinic, Front. Genet., 2012, vol. 3, p. 120.
Liu, N., Landreh, M., Cao, K., et al., The microRNA miR-34 modulates aging and neurodegeneration in Drosophila, Nature, 2012, vol. 482, pp. 519–523.
Soni, K., Choudhary, A., Patowary, A., et al., miR-34 is maternally inherited in Drosophila melanogaster and Danio rerio, Nucleic Acids Res., 2013, vol. 41, no. 8, pp. 4470–4480.
Feng, Y., Huang, W., Meng, W., et al., Heat shock improves sca-1 stem cells survival and directs ischemic cardiomyocytes towards a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway, Stem Cells, 2014, vol. 32, pp. 462–472.
Nikitina, E.A., Medvedeva, A.V., Dolgaya, Yu.F., et al., Involvement of GDNF and LIMK1 and heat shock proteins in Drosophila learning and memory formation, J. Evol. Biochem. Physiol., 2012, vol. 48, nos. 5–6, pp. 529–539.
Sempere, L.F., Sokol, N.S., Dubrovsky, E.B., et al., Temporal regulation of microRNA expression in Drosophila melanogaster mediated by hormonal signals and broad-complex gene activity, Dev. Biol., 2003, vol. 259, no. 1, pp. 9–18.
Kucherenko, M.M. and Shcherbata, H.R., Steroids as external temporal codes act via microRNAs and cooperate with cytokines in differential neurogenesis, Fly (Austin), 2013, vol. 7, no. 3, pp. 173–183.
Chen, G.C., Gajowniczek, P., and Settleman, J., Rho-LIM kinase signaling regulates ecdysone-induced gene expression and morphogenesis during Drosophila metamorphosis, Curr. Biol., 2004, vol. 14, no. 4, pp. 309–313.
Haramati, S., Chapnik, E., Sztainberg, Y., et al., miRNA malfunction causes spinal motor neuron disease, Proc. Natl. Acad. Sci. U.S.A., 2010, vol. 107, pp. 13111–13116.
Yatsenko, A.S. and Shcherbata, H.R., Drosophila miR-9a targets the ECM receptor dystroglycan to canalize myotendinous junction formation, Dev. Cell, 2014, vol. 28, no. 3, pp. 335–348.
Juliano, R., Alam, M.R., Dixit, V., and Kang, H., Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides, Nucleic Acids Res., 2008, vol. 36, pp. 4158–4171.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Savvateeva-Popova, E.A. Nikitina, A.V. Medvedeva, 2015, published in Genetika, 2015, Vol. 51, No. 5, pp. 613–624.
Rights and permissions
About this article
Cite this article
Savvateeva-Popova, E.V., Nikitina, E.A. & Medvedeva, A.V. Neurogenetics and neuroepigenetics. Russ J Genet 51, 518–528 (2015). https://doi.org/10.1134/S1022795415050075
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795415050075