Abstract
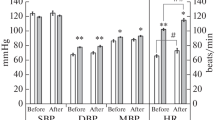
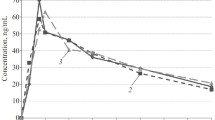
The purpose of this work was to study the pharmacokinetics of the Ca ion antagonist verapamil (a single dose of 80 mg) and hemodynamics in nine volunteers with normal life activities (experiment 1) and on the second day of head-down (–8°) bed rest (experiment 2). The plasma concentration of verapamil was measured using high-performance liquid chromatography with fluorometric detection. Heart rate (HR), cardiac output (CO), stroke volume (SV) and general peripheral vascular resistance (GPVR) were measured using integral rheography according to Tishchenko (Mingograf-410, Russia); blood pressure (BP) was measured by Korotkoff sounds. Analysis of verapamil pharmacokinetics in both experiments showed identity of the averaged curve profiles that did not differ significantly. The pharmacokinetic parameters were comparable. Based on hemodynamics analysis, HR elevation 1 h after verapamil administration was the most common reaction in both experiments; a short CO rise and GPVR decrease were observed in experiment 2 1 h after administration. These hemodynamic shifts were not pathologic and did not deviate from the physiological range in healthy people; changes in verapamil pharmacokinetics were also not detected. These findings provide the grounds to recommend verapamil for rational pharmacotherapy of cardiovascular diseases that may develop in piloted space missions.


Similar content being viewed by others
REFERENCES
Grigoriev, A.I. and Egorov, A.D., General mechanisms of the effect of weightlessness on the human body, in Advances in Space Biology and Medicine, Bonting, S.L., Ed., Amsterdam: Elsevier, 1992, vol. 2, pp. 1–42.
Putcha, L. and Cintron, N.M., Pharmacokinetic consequences of space flight, Ann. N.Y. Acad. Sci., 1991, vol. 618, pp. 615–618.
Goncharov, I.B., Kovachevich, I.V., Repenkova, L.G., et al., Effect of antiorthostatic hypokinesia on acetaminophen pharmacokinetics and its distribution in saliva of healthy volunteers, Pharm. Chem. J., 2009, vol. 43, no. 5, pp. 235–238.
Kukes, V.G., Grachev, S.V., Sychev, D.A., et al., Metabolism lekarstvennykh sredstv. Nauchnye osnovy personalizirovannoi meditsiny: rukovodstvo dlya vrachei (Metabolism of Medical Drugs. Scientific Foundations of Personalized Medicine: Handbook for Physicians), Moscow, 2008.
Agafonov, A.A. and Piotrovskii, V.K., M-IND software for evaluation of pharmacokinetic system parameters by the model-independent method of statistical moments, Khim.-Farm. Zh., 1991, vol. 25, no. 10, pp. 16–19.
Miroshnichenko, I.I., Tyulyaev, I.I., and Zuev, A.P., Biodostupnost’ lekarstvennykh sredstv (Bioavailability of Medical Drugs), Moscow, 2003.
Stadler, P., Leonardi, L., Riesen, W., et al., Cardiovascular effects of verapamil in essential hypertension, Clin. Pharmacol. Ther., 1987, vol. 42, pp. 485–492.
Speders, S., Sosna, J., Schumacher, A., et al., Efficacy and tolerability of Isoptin SR in essential hypertension, Hochdruck, 1988, vol. 8, pp. 3–14.
Golikov, A.P., Ryabinin, V.A., and Sokolov, A.M., The influence of verapamil on blood circulation, Klin. Med., 1986, vol. 64, no. 8, pp. 31–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no obvious or potential conflict of interest related to the publication of this article.
Statement of compliance with standards of research involving humans as subjects. Clinical and physiological studies involving human participants were in accordance with ethical standards of the Ethics Committee, Institute of Medical and Biological Problems, the USSR Ministry of Health. All volunteers were acquainted with study procedure. Informed consent was obtained from all individual participants involved in the study.
Additional information
Translated by M. Novikova
Rights and permissions
About this article
Cite this article
Bogomolov, V.V., Kondratenko, S.N., Polyakov, A.V. et al. Pharmacokinetics of Verapamil and Hemodynamic Indices during Head-down Bed Rest. Hum Physiol 45, 781–785 (2019). https://doi.org/10.1134/S036211971907003X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S036211971907003X