Abstract
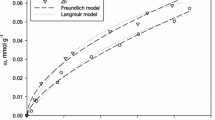
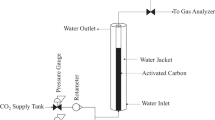
The prediction of adsorption equilibria in multicomponent systems is of prime importance. Therefore, the equilibrium adsorption data for the following multicomponent systems have been studied. The present paper deals with the simultaneous removal phenol (P), 4-chlorophenol (CP), 4-nitrophenol (NP) and catechol (C), resorcinol (R), hydroquinone (HQ) onto modified activated carbon (MAC) from ternary mixtures. The BET surface area of SC was found to be 934 m2/g, whereas BJH adsorption/desorption surface area of pores is 53.03/58.15 m2/g. The equilibrium adsorption data were obtained at different initial concentrations (C0 = 50–1000 mg/L), 12 h contact time, 30°C temperature, MAC dosage of 10 mg/L at solution pH. Equilibrium isotherms for the ternary adsorption of P–CP–NP and C–R–HQ onto MAC have been analyzed by using non-modified Langmuir, modified Langmuir, extended Langmuir, extended Freundlich and Sheindorf–Rebuhn–Sheintuch (SRS) models. The competitive Sheindorf–Rebuhn–Sheintuch (SRS) model fits for both ternary adsorption equilibrium data satisfactorily and adequately.
Similar content being viewed by others
References
Stoilova, A., Krastanov, V., Stanchev, D., Daniel, M., and Alexieva, G. Z., Biodegradation of high amounts of phenol, resorcinol, 2,4-dichlorophenol and 2,6-dimethoxyphenol by Aspergillus awamori cells, Enzyme Microb. Technol., 2006, vol. 39, pp.1036–1041.
Blanco-Martinez, D. A., Giraldo, L., and Moreno-Pirajan, J.C., Effect of the pH in the adsorption and in the immersion enthalpy of monohydroxylated phenols from aqueous solutions on activated carbons, J. Hazard. Mater., 2009, vol. 169, pp. 291–296.
Suresh, S., Srivastava, V.C., and Mishra, I.M., Isotherm, thermodynamics, desorption, and disposal study for the adsorption of catechol and resorcinol onto granular activated carbon, J. Chem. Eng. Data, 2011, vol. 56, no. 4, pp. 811–818.
Othmer, K., Hydroquinone, resorcinol, and catechol, Kirk-Othmer Encyclopedia of Chemical Technology, New York: Wiley, 1981, vol. 13, 3rd ed.
Prager, J.C., Environmental Contaminant Reference Databook, New York: Wiley, 1997, 3rd ed.
International Programme on Chemical Safety (IPCS) Prepared in the Context of Cooperation between the International Programme on Chemical Safety and the European Commission, Geneva: World Health Organisation, 2005.
Guidelines for Drinking-Water Quality, vol. 1: Recommendations, Geneva: World Health Organisation, 1984.
USEPA Federal Register, Washington, DC: United States Environmental Protection Agency, 1987, vol. 52, no. 131, pp. 25861–25962.
Srivastava, V.C., Mall, I.D., and Mishra, I.M., Equilibrium modelling of single and binary adsorption of cadmium and nickel onto bagasse fly ash, Chem. Eng. J., 2006, vol. 117, pp. 79–91.
Suresh, S., Srivastava, V.C., and Mishra, I.M., Adsorptive removal of phenol from binary aqueous solution with aniline and 4-nitrophenol by granular activated carbon, Chem. Eng. J., 2011, vol. 171, no. 3, pp. 997–1003.
Suresh, S., Srivastava, V.C., and Mishra, I.M., Adsorption of hydroquinone in aqueous solution by granulated activated carbon, J. Environ. Eng., 2011, vol. 137, no. 12, pp. 1145–1157.
Suresh, S., Srivastava, V.C., and Mishra, I.M., Study of catechol and resorcinol adsorption mechanism through granular activated carbon characterization, pH and kinetic study, Sep. Sci. Technol., 2011, vol. 46, no. 11, pp. 1750–1766.
Suresh, S. and Keshav, A., Textbook of Separation Processes, New Delhi: Studium, 2012.
Suresh, S., Adsorption of benzoic acid in aqueous solution by bagasse fly ash, J. Inst. Eng. (India): Ser. A, 2012, vol. 93, no. 3, pp. 151–161.
Suresh, S., Srivastava, V.C., and Mishra, I.M., Adsorption of catechol, resorcinol, hydroquinone and its derivatives: A review, Int. J. Energy Environ. Eng., 2012, vol. 32, pp. 1–19.
Suresh, S., Verma, V., Keshav, A., and Soni, A.B., Removal of glycolic acid from aqueous solution using bagasse fly ash, Int. J. Environ. Res., 2012, vol. 6, no. 1, pp. 297–308.
Suresh, S., Srivastava, V.C., and Mishra, I.M., Adsorptive removal of aniline by granular activated carbon from aqueous solutions with catechol and resorcinol, Environ. Technol., 2012, vol. 33, no. 7, pp. 773–781.
Suresh, S., Vijayalakshmi, G., Rajmohan, B., and Subbaramaiah, V., Adsorption of benzene vapor onto activated biomass from cashew nut shell: Batch and column study, Recent Pat. Chem. Eng., 2012, vol. 5, no. 2, pp. 116–133.
Suresh, S., Srivastava, V.C., and Mishra, I.M., Studies of adsorption kinetics and regeneration of aniline, phenol, 4-chlorophenol and 4-nitrophenol by activated carbon, Chem. Ind. Chem. Eng. Q., 2013, vol. 19, no. 2, pp. 195–212.
Suresh, S., Srivastava, V.C., and Mishra, I.M., Removal of 4-nitrophenol from binary aqueous solution with aniline by granular activated carbon using Taguchi’s design of experimental methodology, Theor. Found. Chem. Eng., 2013, vol. 47, pp. 284–290.
Suresh, S. and Sundaramoorthy, S., Green Chemical Engineering: An Introduction to Catalysis, Kinetics and Chemical Processes, Boca Raton, Fla.: CRC, 2015.
Suresh, S., Srivastava, V.C., and Mishra, I.M., Studies of adsorption kinetics and regeneration of aniline, phenol, 4-chlorophenol and 4-nitrophenol by activated carbon, Chem. Ind. Chem. Eng. Q., 2013, vol. 19, no. 2, pp. 195–212.
Bellot, J.C. and Condoret, J.S., Modelling of liquid chromatography equilibrium, Process Biochem., 1993, vol. 28, pp. 365–376.
Yang, R.T., Gas Separation by Adsorption Processes, Boston: Butterworths, 1987.
Sheindorf, C., Rebhum, M., and Sheintuch, M.A., Freundlich-type multicomponent isotherm, J. Colloid Interface Sci., 1981, vol. 79, pp. 136–142.
Srivastava, V.C., Mall, I.D., and Mishra, I.M., Removal of cadmium(II) and zinc(II) metal ions from binary aqueous solution by rice husk ash, Colloids Surf., A, 2008, vol. 312, pp. 172–184.
Brunauer, S., Emmet, P.H., and Teller, F., Adsorption of gases in multimolecular layers, J. Am. Chem. Soc., 1938, vol. 60, p.309.
Fritz, W. and Schluender, E.U., Simultaneous adsorption equilibria of organic solutes in dilute aqueous solutions on activated carbon, Chem. Eng. Sci., 1974, vol. 29, pp. 1279–1282.
Marquardt, D.W., An algorithm for least-squares estimation of nonlinear parameters, J. Soc. Ind. Appl. Math., 1963, vol. 11, pp. 431–441.
Sag, Y., Akcael, B., and Kutsal, T., Ternary biosorption equilibria of chromium(VI), copper(II), and cadmium( II) on Rhizopus arrhizus, Sep. Sci. Technol., 2002, vol. 37, pp. 279–308.
Sag, Y., Akcael, B., and Kutsal, T., Application of multicomponent adsorptionmodels to the biosorption of Cr(II), Cu(II), and Cd(II) ions on Rhizopus arrhizus from ternary metal mixtures, Chem. Eng. Commun., 2003, vol. 190, pp. 797–812.
Chong, K.H. and Volesky, B., Metal biosorption equilibria in a ternary system, Biotechnol. Bioeng., 1996, vol. 49, pp. 629–638.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Suresh, S., Srivastava, V.C. & Mishra, I.M. Equilibrium Modeling of Ternary Adsorption of Phenols onto Modified Activated Carbon. Theor Found Chem Eng 52, 271–285 (2018). https://doi.org/10.1134/S0040579518020173
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579518020173