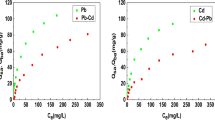
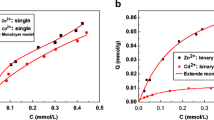
Abstract
This paper describes an experimental and modeling analysis of cadmium and zinc adsorption from aqueous solutions in single and binary systems on activated carbon. Batch tests were performed at constant pH (6.8 ± 0.3) and temperature (20 °C), in order to assess the adsorption capacity in experimental conditions simulating real wastewater. In particular, binary tests were carried out at same pH and temperature, with different initial concentration ratios of the two analytes (\(C_{Zn}^{0}\):\(C_{Cd}^{0}\)), in order to emphasize the competition phenomena while minimizing the effect of pH. Experimental results indicate that zinc is adsorbed to a greater extent than cadmium, both in single and binary systems. In binary systems, zinc adsorption capacity is not influenced by the presence of cadmium, regardless of its concentration. On the contrary, an increase in zinc concentration in solution brings about a decrease in cadmium adsorption capacity. The experiments were interpreted by using both the rigorous and the extended Langmuir multicomponent adsorption isotherms and the Vacancy Solution Theory. The latter provided a satisfactory and simultaneous description of the experimental data for both zinc and cadmium adsorption, also accounting for the possible interactions between the two analytes and between the solvent (water) and the single analyte.








Similar content being viewed by others
References
Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for zinc. U.S. Public Health Service, U.S. Department of Health and Human Services, Atlanta (2005)
Babić, B.M., Milonjić, S.K., Polovina, M.J., Čupić, S., Kaludjerović, B.V.: Adsorption of zinc, cadmium and mercury ions from aqueous solutions on an activated carbon cloth. Carbon 40, 1109–1115 (2002)
Balsamo, M., Di Natale, F., Erto, A., Lancia, A., Montagnaro, F., Santoro, L.: Steam- and carbon dioxide-gasification of coal combustion ash for liquid phase cadmium removal by adsorption. Chem. Eng. J. 207–208, 66–71 (2012)
Benjamin, M.: Water Chemistry. McGraw Hill, New York (2002)
Bhatnagar, A., Sillanpää, M.: Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—a review. Chem. Eng. J. 157, 277–296 (2010)
Di Natale, F., Erto, A., Lancia, A., Musmarra, D.: A descriptive model for metallic ions adsorption from aqueous solutions onto activated carbons. J. Hazard. Mater. 169, 360–369 (2009)
Di Natale, F., Erto, A., Lancia, A., Musmarra, D.: Equilibrium and dynamic study on hexavalent chromium adsorption onto activated carbon. J. Hazard. Mater. 281, 47–55 (2015)
El Shafei, G.M.S., El Sherbiny, I.M.A., Darwish, A.S., Philip, C.A.: Silkworms’ feces-based activated carbons as cheap adsorbents for removal of cadmium and methylene blue from aqueous solutions. Chem. Eng. Res. Des. 92, 461–470 (2014)
Erto, A., Andreozzi, R., Di Natale, F., Lancia, A., Musmarra, D.: Experimental and statistical analysis of trichloroethylene adsorption onto activated carbon. Chem. Eng. J. 156, 353–359 (2010a)
Erto, A., Andreozzi, R., Lancia, A., Musmarra, D.: Factors affecting the adsorption of trichloroethylene onto activated carbons. Appl. Surf. Sci. 256, 5237–5242 (2010b)
Erto, A., Lancia, A., Musmarra, D.: A modelling analysis of PCE/TCE mixture adsorption based on Ideal Adsorbed Solution Theory. Sep. Purif. Technol. 80, 140–147 (2011)
Erto, A., Lancia, A., Musmarra, D.: A real adsorbed solution theory model for competitive multicomponent liquid adsorption onto granular activated carbon. Microp. Mesopor. Mater. 154, 45–50 (2012)
Fagundes-Klen, M.R., Ferri, P., Martins, T.D., Tavares, C.R.G., Silva, E.A.: Equilibrium study of the binary mixture of cadmium–zinc ions biosorption by the Sargassum filipendula species using adsorption isotherms models and neural network. Biochem. Eng. J. 34, 136–146 (2007)
Fu, F., Wang, Q.: Removal of heavy metals ions from wastewaters: a review. J. Environ. Manag. 92(3), 407–418 (2011)
González, P.G., Pliego-Cuervo, Y.B.: Adsorption of Cd(II), Hg(II) and Zn(II) from aqueous solution using mesoporous activated carbon produced from Bambusa vulgaris striata. Chem. Eng. Res. Des. 92, 2715–2724 (2014)
International Agency for Research on Cancer (IARC). Cadmium and cadmium compounds (Group1), IARC monographs, Lyon (2012)
Kumar, K.V., de Monteiro Castro, M.C., Martinez-Escandell, M., Molina-Sabio, M., Rodriguez-Reinoso, F.: Adsorption on heterogeneous surfaces: site energy distribution functions from Fritz–Schlüender isotherms. ChemPhysChem 11, 2555–2560 (2010)
Le Van, M.D., Vermeulen, T.: Binary Langmuir-like and Freundlich isotherms for ideal adsorbed solutions. J. Phys. Chem. 85, 4 (1981)
Leyva-Ramos, R., Bernal-Jacome, L.A., Guerrero-Coronado, R.M., Fuentes-Rubio, L.: Competitive adsorption of Cd(II) and Zn(II) from aqueous solution onto activated carbon. Sep. Sci. Technol. 36, 3673–3687 (2001)
Lu, C., Chiu, H.: Adsorption of zinc(II) from water with purified carbon nanotubes. Chem. Eng. Sci. 61(4), 1138–1145 (2006)
Macias-Garcia, A., Gómez-Serrano, V., Alexandre-Franco, M.F., Valenzuela-Calahorro, C.: Adsorption of cadmium by sulphur dioxide treated activated carbon. J. Hazard. Mater. 103, 141–152 (2003)
Mohan, D., Singh, K.P.: Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—an agricultural waste. Water Res. 36, 2304–2318 (2002)
Molino, A., Erto, A., Natale, F.D., Donatelli, A., Iovane, P., Musmarra, D.: Gasification of granulated scrap tires for the production of syngas and a low-cost adsorbent for Cd(II) removal from wastewaters. Ind. Eng. Chem. Res. 52, 12154–12160 (2013)
Nadeem, M., Shabbir, M., Abdullah, M.A., Shah, S.S., McKay, G.: Sorption of cadmium from aqueous solution by surfactant-modified carbon adsorbents. Chem. Eng. J. 148, 365–370 (2009)
Ncibi, M.C., Altenor, S., Seffen, M., Brouers, F., Gaspard, S.: Modelling single compound adsorption onto porous and non-porous sorbents using a deformed Weibull exponential isotherm. Chem. Eng. J. 145(2), 196–202 (2008)
Noh, J.S., Schwarz, J.A.: Effect of HNO3 treatment on the surface acidity of activated carbons. Carbon 28, 675–682 (1990)
Nordberg, G.F., Fowler, B.A., Nordberg, M.: Chapter 1—toxicology of metals: overview, definitions, concepts, and trends. In: Nordberg, G.F., Fowler, B.A. (eds.) Handbook on the Toxicology of Metals, 4th edn, pp. 1–12. Academic Press, San Diego (2015)
Pardo-Botello, R., Fernández-González, C., Pinilla-Gil, E., Cuerda-Correa, E.M., Gómez-Serrano, V.: Adsorption kinetics of zinc in multicomponent ionic systems. J. Colloid Interface Sci. 277, 292–298 (2004)
Cazón, J.P., Viera, M., Donati, E., Guibal, E.: Zinc and cadmium removal by biosorption on Undaria pinnatifida in batch and continuous processes. J. Environ. Manage. 129, 423–434 (2013)
Rangel-Mendez, J.R., Streat, M.: Adsorption of cadmium by activated carbon cloth: influence of surface oxidation and solution pH. Water Res. 36, 1244–1252 (2002)
Rudzinski, W., Panczyk, T.: The Langmuirian adsorption kinetics revised: a farewell to the XXth century theories? Adsorption 8, 23–34 (2002)
Srivastava, V.C., Mall, I.D., Mishra, I.M.: Removal of cadmium(II) and zinc(II) metal ions from binary aqueous solution by rice husk ash. Colloid Surface A 312, 172–184 (2008)
Stumm, W., Morgan, J.J.: Aquatic Chemistry. Wiley, New York (1996)
Suwanayuen, S., Danner, R.P.: A gas adsorption isotherm equation based on vacancy solution theory. AIChE J. 26, 68–76 (1980a)
Suwanayuen, S., Danner, R.P.: Vacancy solution theory of adsorption from gas mixtures. AIChE J. 26, 76–83 (1980b)
Vocciante, M., Trofa, M., D’Auria, T., Giraldo, L., Rodriguez-Estupiñan, P., Moreno Pirajan, J.C., Erto, A.: Experimental and simulation-based studies of cadmium and nickel adsorption from wastewater. J. Clean. Prod. 77, 35–46 (2014)
Zhu, C., Luan, Z., Wang, Y., Shan, X.: Removal of cadmium from aqueous solutions by adsorption on granular red mud (GRM). Sep. Purif. Technol. 57, 161–169 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erto, A., Di Natale, F., Musmarra, D. et al. Modeling of single and competitive adsorption of cadmium and zinc onto activated carbon. Adsorption 21, 611–621 (2015). https://doi.org/10.1007/s10450-015-9712-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9712-6