Abstract
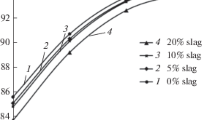
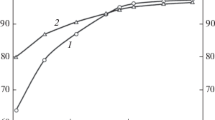
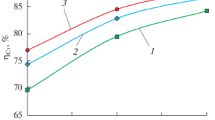
Thermodynamic simulation in a liquid state is performed to study the possibility of reduction of the elements of the multicomponent Cr2O3–FeO–CaO–SiO2–MgO–Al2O3 system. The parameters of the initial state of the system are as follows: the temperature range (t) is 1500–1800°C, the step is 100°C, the total system pressure is 0.1 MPa, and the amount of nitrogen is 2.24 m3. The aim of the work is to study the influence of the amount of gas-cleaning dust added to an ore on the reduction of the elements in making ferrochrome in the given temperature range. The oxide system composition corresponds to the chromium ore ((%) 38 Cr2O3, 11.1 FeO, 0.17 CaO, 15 SiO2, 29.7 MgO, 6 Al2O3) and the gas-cleaning dust (15.5 Cr2O3, 7.5 FeO, 0.8 CaO, 67.3 SiO2, 7.2 MgO, 1.7 Al2O3) that are used to produce medium-carbon ferrochrome. The dust content in a mixture was 0, 5, 10, and 20%. Carbon was used as a reducing agent. The consumption of the reducing agent is increased by 10% of the stoichiometry for the reduction of Fe and Cr and by 8% of the metal mass for the formation of iron, chromium, and silicon carbides. The simulation is carried out using HSC Chemistry 6.12 (Outokumpu, Finland) software package. The thermodynamic data of the CrO(II) compound are introduced into the database, and the thermochemical characteristics of the compounds CaCr2O4, SiC, Cr3C2, Cr7C3, Cr23C6, and Fe3C, which exist in the database, are refined. It was determined that increase in the melt temperature from 1500 to 1700°C is found to increase the degree of chromium reduction (ηCr) from 90.2 to 94.8% at various dust contents in the system. An increase in the dust content in a mixture from 0 to 20% decreases ηCr. The maximum value of ηCr is characteristic of a dust-free system: it is 94.8% at (CaO + MgO)/(SiO2) = 2.0 and t = 1700°C. The chemical compositions of the metal and slag melts are determined. The amount of [Cr] = 64–65.6% at a melt temperature of 1700°C. The simulation results can be used to analyze the reduction processes occurring in chromium-containing systems and alloy production technologies.



Similar content being viewed by others
REFERENCES
L. D. Nikitin, N. G. Dyachok, A. V. Vashchenko, A. I. Shentsov, and V. I. Kutran’, Steel Transl. 49 (7), 472–477 (2019). https://doi.org/10.3103/S0967091219070064
V. V. Temnikov, E. G. Kalimulina, and B. S. Tleugabulov, “Analysis of the formation and processing of the metallurgical wastes in AO EVRAZ NTMK,” Chern. Metally, No. 7, 32–37 (2018).
I. V. Butorina and M. V. Butorina, “Review of the waste disposal technologies in the mining and metallurgical industry,” Chern. Metally, No. 12, 44–49 (2018).
B. L. Demin, Yu. V. Sorokin, L. A. Smirnov, and E. N. Shcherabakov, “Stabilization of decaying ferroalloy and steelmaking slags,” in Proceedings of Conference on Prospects for the Development of Metallurgy and Mechanical Engineering Using Completed Fundamental Research and R&D: Ferroalloys (Yekaterinburg, 2018), pp. 342–345.
B. L. Demin, Yu. V. Sorokin, L. A. Smirnov, and E. N. Shcherbakov, “Existing methods of processing and directions of use of ferroalloy slags,” in Proceedings of Conference on Prospects for the Development of Metallurgy and Mechanical Engineering Using Completed Fundamental Research and R&D: Ferroalloys (Yekaterinburg, 2018), pp. 346–350.
State Report on the State and Environmental Protection of the Russian Federation in 2015. Cited March 17, 2020. http://www.mnr.gov.ru/docs/o_sostoyanii_i_ ob_okhrane_okmzhayushchey_sredy_rossiyskoy_federatsii/gosudarstvennyy_doklad_o_sostoyanii_i_ob_okhrane_okruzhayushchey_sredy_rossiyskoy_federatsii_v_2016_/.
A. V. Zhdanov, V. I. Zhuchkov, V. Ya. Dashevskii, and L. I. Leont’ev, Metallurgist 58 (11–12), 1064–1070 (2015). https://doi.org/10.1007/s11015-015-0041-5
V. I. Zhuchkov, O. V. Zayakin, and A. V. Sychev, Russ. Metall. (Metally), No. 6, 662–666 (2020). https://doi.org/10.1134/S003602952006018X
I. V. Kushnerev, M. B. Orzhekh, B. B. Libanov, S. A. Koroteev, A. A. Platonov, and V. V. Plyukhin, “Stabilization of the steel ladle treatment slag from silica dissolution,” Novye Ogneupory, No. 4, 44 (2018).
V. V. Kozlov, A. P. Shevchik, S. A. Suvorov, N. V. Arbozova, and D. V. Kuznetsov, Refractories and Industrial Ceramics 59 (5), 502–506 (2019). https://doi.org/10.1007/s11148-019-00262-9
Zhang Yang-ling, Liu Y., Wei Wen-jie, Int. J. Min., Metall. Mater. 20 (10), 931–940 (2013). https://doi.org/10.1007/s12613-013-0817-1
R. E. Kryukov, V. F. Goryushkin, Yu. V. Bendre, L. P. Bashchenko, and N. A. Kozyrev, Steel Transl. 49, 843–847 (2019). https://doi.org/10.3103/S0967091219120052
I. E. Doronin and A. G. Svyazhin, Metallurgist 57 (1–2), 41–48 (2013). https://doi.org/10.1007/s11015-013-9688-y
M. Leuchtenmüller, J. Antrekowitsch, and S. Steinlechner, Metall. Mater. 50 (5), 2221–2228 (2019). https://doi.org/10.1007/s11663-019-01649-2
I. V. Bondarenko and E. A. Tastanov, Metallurgist 62 (11–12), 1213–1218 (2019). https://doi.org/10.1007/s11015-019-00776-0
A. G. Kaliakparov, S. R. Baltabaev, V. M. Strakhov, A. A. Mukhtar, Metallurgist 61 (9–10), 765–769 (2017). https://doi.org/10.1007/s11015-018-0561-x
N. A. Kozyrev, A. A. Usol’tsev, A. N. Prudnikov, R. E. Kryukov, and A. R. Mikhno, “Studying the properties of a powder wire based on ferrochrome gas purification dust,” Chern. Metall. 75 (3), 365–372 (2019). https://doi.org/10.32339/0135-5910-2019-3-365-372
V. I. Zhuchkov, V. A. Salina, O. V. Zayakin, and A. V. Sychev, “Thermodynamic simulation of the influence of the oxide system temperature and composition on chromium reduction,” in Proceedings of Conference on Prospects for the Development of Metallurgy and Mechanical Engineering Using Completed Fundamental Research and R&D (UrO RAN, Yekaterinburg, 2020). pp. 212–215.
V. A. Salina, V. I. Zhuchkov, and A. V. Sychev, “Thermodynamic simulation of the carbothermic reduction of chromium from the Cr2O3–FeO–CaO–SiO2–MgO–Al2O3 oxide system,” Rasplavy, No. 6, 608–615 (2020).
Z. T. Bai, Z. A. Zhang, M. Guo, X. M. Hou, and M. Zhang, Mater. Res. Innov., No. 5, 113–118 (2015). https://doi.org/10.1179/1432891714z.0000000001065
T. Wu, F. Yuan, and Ya. Zhang, ISIJ Int. 58 (2), 367–369 (2018). https://doi.org/10.2355/isijinternational.ISIJINT-2017-558
N. Sahu, A. Biswas, U. K. Gajanan, Mineral Proc. Extract. Metall. Review 37 (4), 211–219 (2016). https://doi.org/10.1080/08827508.2016.1168415
A. Roine, Outokumpu HSC Chemistry for Windows. Chemical Reactions and Equilibrium Software with Extensive Thermochemical Database (Outokumpu research OY, Pori, 2002).
V. P. Glushko, Thermal Constants of Substances (AN SSSR, Moscow, 1970–1972, 1974, 1979).
M. A. Ryss, Production of Ferroalloys (Metallurgiya, Moscow, 1985).
M. I. Gasik, N. P. Lyakishev, and B. I. Emlin, Theory and Technology of Ferroalloy Production (Metallurgiya, Moscow, 1988).
M. I. Gasik and N. P. Lyakishev, Metallurgy of Chromium (ELIZ, Moscow, 1999).
I. V. Ryabchikov, V. G. Mizin, and K. I. Yarovoi, Steel Transl. 43 (6), 379–382 (2013). https://doi.org/10.3103/S096709121306017X
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 18-29-24027.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Salina, V.A., Zhuchkov, V.I. Study of the Carbothermic Reduction of the Elements of the Cr2O3–FeO–CaO–SiO2–MgO–Al2O3 System by Thermodynamic Simulation. Russ. Metall. 2022, 128–133 (2022). https://doi.org/10.1134/S0036029522020161
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029522020161