Abstract
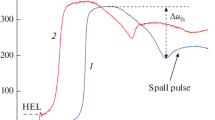
The explosive solidification of supercooled liquids is analyzed using heating and cooling thermograms of various substances. As proof of the phenomenon of explosive solidification, thermograms in the temperature–time coordinates are presented for tellurium, bismuth, water, and acetic acid. A schematic cooling curve is used to demonstrate the structural changes occurring in a liquid phase and the stages of solidification, namely, the formation of crystal nuclei, their coalescence, and subsequent isothermal solidification. Explosive solidification at the second stage is characterized by a rapid temperature rise from a supercooling region to the melting temperature at the rates exceeding the cooling rate (i.e., heat removal) by 2–3 orders of magnitude. An attempt is made to explain the phenomenon of explosive solidification in terms of the well-known postulates of the cluster-coalescence model of solidification and the theory of chain reactions. The calculations demonstrate that the critical nucleus sizes are comparable with the unit cell sizes of the corresponding crystal lattices and the nucleus formation energy is comparable with the intermolecular bond energies. Based on the calculations of the heat released during nucleus formation and the interfacial surface energy released during the coalescence of crystal nuclei, we advance a hypothesis that crystalline clusters and stable crystal nuclei can serve as the “building blocks” of crystal growth along with molecules. The energy released during the formation of nuclei and their coalescence is shown to be equivalent to electromagnetic radiation quanta and to generate new centers of solidification, multiplication, and coalescence of nuclei. The calculations demonstrate that the energy released during the coalescence of many nuclei is high enough to quickly heat a substance from the supercooling region to the melting temperature. By analogy with the well-known thermal explosion diagram, we plotted and analyzed a similar diagram for the time dependence of the heat release and the heat removal during explosive solidification. The critical values of the beginning of the explosive process and the cooling rate of a liquid phase are found. The final stage of equilibrium solidification after explosive solidification is estimated.



Similar content being viewed by others
REFERENCES
V. A. Shklovskii and V. M. Kuz’menko, “Explosive solidification of amorphous substances,” Usp. Fiz. Nauk 57 (2), 311–338 (1989).
V. P. Skripov and V. P. Koverda, Spontaneous Solidification of Supercooled Liquids (Nauka, Moscow, 1984).
V. D. Aleksandrov, Kinetics of Nucleation and Mass Solidification of Supercooled Liquids and Amorphous Media (Donbass, Donetsk, 2011).
V. D. Aleksandrov and A. A. Barannikov, “Cyclic thermal analysis of the influence of the thermal history of tin and lead droplets on their solidification,” Khim. Fiz. 17 (10), 140–147 (1998).
V. D. Aleksandrov and V. I. Petrenko, “New exo- and endothermic effects in the tellurium melt detected by CTA,” Rasplavy 2 (5), 29–34 (1988).
V. D. Aleksandrov and A. A. Barannikov, “Thermal effects induring the solidification of water droplets under natural conditions,” Zh. Fiz. Khim. 74 (4), 595–599 (2000).
V. D. Aleksandrov, V. A. Postnikov, and N. V. Shchebetovskaya, “Presolidification supercooling of acetic acid drops,” Vestn. Novgorod. Gos. Univ., No. 61, 64–68 (2014).
A. A. Shibkov, M. A. Zheltov, A. A. Korolev, and A. V. Maiorov, “Electromagnetic and acoustic emission during explosive solidification of a supercooled water drop,” Vestn. Tambov Gos. Univ, No. 4, 395–398 (1999).
Ya. I. Dutchak, X-ray Diffraction of Liquid Metals (Vyshcha Shkola, Lviv, 1977).
A. D. Skryshevskii, Structural Analysis of Liquids and Amorphous Bodies (Vyssh. Shkola, Moscow, 1980).
B. Chalmers, Principles of Solidification (Wiley, New York, 1968).
I. H. Perepezko, “Nucleation in undercooled liquids,” Mater. Sci. Eng. 1 (1), 125–135 (1984).
V. D. Aleksandrov and E. A. Pokintelitsa, “Method for calculating the size of nuclei during homogeneous solidification from a supercooled liquid,” Zh. Fiz. Khim. 90 (9), 1385–1388 (2016).
M. E. Drits, Properties of Elements: A Handbook (Metallurgiya, Moscow, 1985).
N. N. Semenov, Chain Reactions (Goskhimtekhizdat, Leningrad, 1934).
N. S. Akulov, Theory of Chain Reactions (Gostekhizdat, Moscow, 1951).
K. K. Andreev and A. F. Belyaev, Theory of Explosives (Oborongiz, Moscow, 1960).
A. V. Totai and O. G. Kazakov, Theory of Combustion and Explosion (Yurait, Moscow, 2018).
M. Volmer, Kinetics of Phazenbildung (Steinkopf, Dreseden, 1982).
J. W. Christian, The Theory of Transformations in Metals and Alloys. Part 1. Equilibrium and General Kinetic Theory (Pergamon, 1975).
B. Wunderlich, Macromolecular Physics. Vol. 2 Crystal Nucleation, Annealing (Academic, 1975).
A. G. Stromberg and D. P. Semchenko, Physical Chemistry (Vysshaya Shkola, Moscow, 2001).
V. D. Aleksandrov, A. P. Zozulya, and A. Yu. Sobolev, “Analysis of the kinetics of nucleation and mass solidification of a supercooled melt in accordance with fusibility thermograms,” Rasplavy, No. 3, 350–358.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Aleksandrov, V.D., Frolova, S.A. Coalescence Mechanism of the Explosive Solidification of Supercooled Liquids. Russ. Metall. 2021, 913–918 (2021). https://doi.org/10.1134/S0036029521080036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029521080036