Abstract
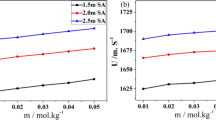
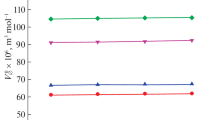
This investigation is made to predict and assess the interactions of two nonessential amino acids (L-aspartic acid and L-glutamic acid) with a food additive, sodium acetate in aqueous medium. Volumetric and viscometric methods are chosen to determine various physicochemical and thermodynamic parameters important for understanding the interactional behavior of the amino acids with sodium acetate and the effect of these interactions on 3D network structure of water. Analysis of parameters like apparent molar volume, limiting apparent molar volume (\(V_{\phi }^{0}\)), limiting apparent molar expansibility (\(E_{\phi }^{0}\)) and viscosity coefficients (\({{B}_{{{\text{J}}}}}\) and \({{A}_{{\text{F}}}}\)) indicates towards \(({{V}_{\phi }})\) strong ion-solvent interactions in the experimental solutions. Variations of \(E_{\phi }^{0}\) and \({{B}_{{{\text{J}}}}}\) with temperature substantiate the kosmotropic character of the amino acids in presence of SA. Evolution of high energy transition state in order to initiate viscous flow of solutions is evidenced by the positive activation enthalpy and free energy of activation of viscous flow.




Similar content being viewed by others
REFERENCES
R. Gaba, A. Pal, D. Sharma, H. Kumar, and A. Kumar, J. Mol. Liq. 279, 711 (2019). https://doi.org/10.1016/j.molliq.2019.01.094
Z. Yan, J. Wang, W. Kong, and J. Lu, Fluid Phase Equilib. 215, 143 (2004). https://doi.org/10.1016/j.fluid.2003.07.001
H. Kumar, A. Katal, and P. K. Sharma, J. Chem. Eng. Data 65 (1473), 1487 (2020). https://doi.org/10.1021/acs.jced.9b00902
R. Gaba, A. Pal, H. Kumar, and D. Sharma, Navjot, J. Mol. Liq. 242, 739 (2017). https://doi.org/10.1016/j.molliq.2017.07.058
H. Kumar, R. Sharma, V. Kumar, and N. Al Masoud, J. Chem. Thermodyn. 158, 106452 (2021). https://doi.org/10.1016/j.jct.2021.106452
S. Sharma, S. Sharma, J. Singh, M. Singh, A. K. Sharma, and M. Sharma, J. Chem. Thermodyn. 167, 106696 (2022). https://doi.org/10.1016/j.jct.2021.106696
M. A. Jamal, T. A. Sajid, M. Saeed, B. Naseem, and M. Muneer, J. Mol. Liq. 360, 119510 (2022). https://doi.org/10.1016/j.molliq.2022.119510
A. Hussain and A. M. Khan, J. Mol. Liq. 365, 120172 (2022). https://doi.org/10.1016/j.molliq.2022.120172
A.Hussain, A. D. Shuaibu, A. J. Shaikh, and A. M. Khan, J. Mol. Liq. 347, 118003 (2022). https://doi.org/10.1016/j.molliq.2021.118003
K. Dhal, S. Singh, and M. Talukdar, Mater. Today: Proc. 67, 1218 (2022). https://doi.org/10.1016/j.matpr.2022.08.290
K. Dhal, S. Singh, and M. Talukdar, J. Mol. Liq. 368, 120761 (2022). https://doi.org/10.1016/j.molliq.2022.120761
K. Dhal, S. Singh, and M. Talukdar, J. Mol. Liq. 361, 119578 (2022). https://doi.org/10.1016/j.molliq.2022.119578
K. Dhal, S. Singh, and M. Talukdar, J. Mol. Liq. 352, 118659 (2022). https://doi.org/10.1016/j.molliq.2022.118659
U. N. Dash, S. Mishra, and B. Samantray, Egyp. J. Chem. 53 (163), 176 (2010). https://doi.org/10.21608/EJCHEM.2010.1210
T. S. Banipal, K. Singh, and P. K. Banipal, J. Solution Chem. 36, 1635 (2007). https://doi.org/10.1007/s10953-007-9212-8
M. A. Jamal, B. Naseem, M. K. Khosa, M. Muneer, and J. H. Khan, J. Mol. Liq. 237, 14 (2017). https://doi.org/10.1016/j.molliq.2017.04.073
D. Kumar, S. S. Shah, T. Sharma, D. Singh, and R. K. Bamezai, Chem. Thermodyn. Therm. Anal. 8, 100090 (2022). https://doi.org/10.1016/j.ctta.2022.100090
G. R. Hedwig, J. Sol. Chem. 17, 383 (1988).
A. Klofutar, J. Horvat, and D. Rudan-Tasič, Acta Chim. Slov. 53, 274 (2006).
M. A. Jamal, M. Rashad, M. K. Khosa, I. A. Bhatti, and K. M. Zia, Food Chem. 153, 140 (2014). https://doi.org/10.1016/j.foodchem.2013.12.039
J. L. Richards, J. Chem. Educ. 70, 685 (1993). https://doi.org/10.1021/ed070p685
S. Chauhan, M. S. Chauhan, J. Jyoti, and Rajni, J. Mol. Liq. 148, 24 (2009). https://doi.org/10.1016/j.molliq.2009.05.002
A. Pal and S. Kumar, J. Chem. Sci. 117, 267 (2005). https://doi.org/10.1007/BF02709297
M. Clugston and R. Fleming, Advanced Chemistry (Oxford Univ. Press, 2000).
R. Rani, A. Kumar, and R. K. Bamezai, J. Mol. Liq. 224, 1142 (2016). https://doi.org/10.1016/j.molliq.2016.10.063
H. Kumar, M. Singla, and R. Jindal, J. Mol. Liq. 199, 385 (2014). https://doi.org/10.1016/j.molliq.2014.09.038
O. S. Lawal, Food Chem. 95, 101 (2006). https://doi.org/10.1016/j.foodchem.2004.12.041
A. Feakins, D. Freemantle, and K. G. Lawrence, J. Chem. Soc. Faraday Trans. 70, 795 (1974). https://doi.org/10.1039/F19747000795
F. Salimi and F. Frouzesh, J. Chem. Thermodyn. 126, 22 (2018). https://doi.org/10.1016/j.jct.2018.06.008
X. Jiang, C. Zhu, and Y. Ma, J. Chem. Thermodyn. 71, 50 (2014). https://doi.org/10.1016/j.jct.2013.11.002
X. Ren, C. Zhu, and Y. Ma, J. Chem. Thermodyn. 93, 179 (2016). https://doi.org/10.1016/j.jct.2015.10.002
ACKNOWLEDGMENTS
We, the authors of this article, are extremely thankful to Department of Chemistry, ITER, Siksha О Anusandhan Deemed to be University for the facilities extended to us for completion of the experimental work presented here.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhal, K., Singh, S. & Talukdar, M. Investigation on Volumetric and Viscometric Properties of Aqueous Solutions of L-Aspartic Acid and L-Glutamic Acid in Presence of Sodium Acetate. Russ. J. Phys. Chem. 97, 3013–3027 (2023). https://doi.org/10.1134/S0036024423130137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423130137