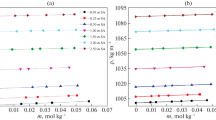
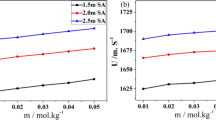
Abstract
Study of some physicochemical properties of l-aspartic aid in water and aqueous ammonium acetate has been undertaken for this investigation. Density, viscosity and conductivity of the solutions were measured at different temperatures. Volumetric parameters such as apparent molar volume \(({V}_{\Phi })\), partial molar volume \(({V}_{\Phi }^{0})\), partial molar expansibility \(({E}_{\Phi }^{0})\) and Hepler’s constant \(({{\partial }^{2}{V}_{\Phi }^{0}/{\partial T}^{2})}_{{p}})\) and viscometric parameters such as relative viscosity\({(\eta }_{r })\), viscosity coefficients \({(B}_{J }\mathrm\,{\text{and} }\,{ A}_{F})\), Gibbs free energy of activation of viscous flow of solvent and solute \({(\Delta \mu }_{1}^{\#,0}\mathrm\,\text{ and }\,{\Delta \mu }_{2}^{\#,0})\) respectively) and corresponding change in enthalpy \({\Delta H}_{2}^{\#,0}\) and entropy \({\Delta S}_{2}^{\#,0}\) have been derived from the experimentally measured density and viscosity values. Variation of these parameters with concentration and temperature was analysed in the light of ion–ion and ion–hydrophilic interactions. The possibility of ion pair formation and its effect on migration of charged particles in solution have been determined by analysing the molar conductance (Λm), association constant \({(K}_\text{A})\), Walden product and thermodynamic functions. These parameters were qualitatively correlated with changes in structure of water that occurs when l-Aspartic acid interacts with ammonium acetate in aqueous media.





Similar content being viewed by others
References
Sembira-Nahum, Y., Apelblat, A., Manzurola, E.: Volumetric properties of aqueous solutions of l-glutamic acid and magnesium-l-glutamate. J. Solution Chem. 37, 391–401 (2008). https://doi.org/10.1007/s10953-007-9245-z
Deosarkar, S.D., Arsule, A.D., Sawale, R.T., Pingle, V.G.: Volumetric and viscometric studies of molecular interactions in systems containing tartaric acid in water/aqueous-l-arginine solutions at 303.15 K. J. Mol. Liq. 323, 114925 (2021). https://doi.org/10.1016/j.molliq.2020.114925
Kumar, H., Kumar, V., Sharma, S., Katal, A., Alothman, A.A.: Volumetric and acoustic properties of amino acids l-leucine and l-serine in aqueous solution of ammonium dihydrogen phosphate (ADP) at different temperatures and concentrations. J. Chem. Thermodyn. 155, 106350 (2021). https://doi.org/10.1016/j.jct.2020.106350
Arsule, A.D., Sawale, R.T., Deosarkar, S.D.: Temperature-dependent volumetric and ultraacoustic studies of α-amino acids in aqueous acetylsalicylic acid drug solutions. J. Mol. Liq. 275, 478–490 (2019). https://doi.org/10.1016/j.molliq.2018.10.122
Rani, R., Kumar, A., Sharma, T., Bamezai, R.K.: Volumetric, acoustic and transport properties of ternary solutions of l-serine and l-arginine in aqueous solutions of thiamine hydrochloride at different temperatures. J. Chem. Thermodyn. 135, 260–277 (2019). https://doi.org/10.1016/j.jct.2019.03.039
Sharma, S.K., Thakur, A.: Volumetric, acoustic and viscometric studies of solute–solute and solute–solvent interactions of glycyl-glycyl-glycine in aqueous tartaric acid at different temperatures. J. Mol. Liq. 322, 114527 (2021). https://doi.org/10.1016/j.molliq.2020.114527
Ankita, N.A.K.: Study on the interactions of drug isoniazid in aqueous d-xylose/l-arabinose solutions at different temperatures using volumetric, acoustic and viscometric approaches. J. Mol. Liq. 298, 112086 (2020). https://doi.org/10.1016/j.molliq.2019.112086
Savaroglu, G., Ildaser, A.C.: Volumetric interaction coefficients for some nucleosides in aqueous solution at T=298.15K. Thermochim. Acta 582, 86–93 (2014). https://doi.org/10.1016/j.tca.2014.03.004
Gaba, R., Pal, A., Sharma, D., Kumar, H., Kumar, A.: Molecular interactions of some non-essential amino acids in aqueous solutions of 1-methylimidazolium chloride at different temperatures. J. Mol. Liq. 279, 711–718 (2019). https://doi.org/10.1016/j.molliq.2019.01.094
Gaba, R., Malhotra, J., Pal, A., Sharma, D., Kumar, H.: Molecular interactions of l-glutamic acid and l-aspartic acid in aqueous solutions 1-heptyl-3-methyl imidazoliumtetrafluoroborate [C7mim][BF4] at different temperatures. J. Mol. Liq. 322, 114971 (2021). https://doi.org/10.1016/j.molliq.2020.114971
Dhal, K., Singh, S., Talukdar, M.: Elucidation of molecular interactions of aspartic acid with aqueous potassium sorbate and sodium benzoate: Volumetric, viscometric and FTIR spectroscopic investigation. J. Mol. Liq. 361, 119578 (2022). https://doi.org/10.1016/j.molliq.2022.119578
Dhal, K., Singh, S., Talukdar, M.: Volumetric, viscometric and spectroscopic studies of molecular interactions of glutamic acid with potassium sorbate and sodium benzoate in aqueous medium at T = 293.15–313.15 K. J. Mol. Liq. 361, 119578 (2022). https://doi.org/10.1016/j.molliq.2022.119578
Talukdar, M., Singh, S., Dehury, S.K.: A review on conductometric studies of electrolytes in mixed solvent systems to understand ion–ion and ion–solvent interactions. Biointerface Res. Appl. Chem. 10(2), 5332–5337 (2020). https://doi.org/10.33263/BRIAC102.332337
Kell, G.S.: Density, thermal expansibility and compressibility of liquid water from 00 to 1500 C: correlation and tables for atmospheric pressure and saturation reviewed and expressed on 1968 temperature scale. J. Chem. Eng. Data 20(1), 97–105 (1975). https://doi.org/10.1021/je60064a005
Rajput, P., Singh, H., Kumar, A.: Volumetric, ultrasonic and viscometricbehavior of nucleosides (uridine and cytidine) in aqueous l-ascorbic acid solutions at different temperatures. J. Chem. Thermodyn.171, 106805 (2022). https://doi.org/10.1016/j.jct.2022.106805
Richards, J.L.: Viscosity and the shapes of macromolecules: a physical chemistry experiment using molecular-level models in the interpretation of macroscopic data obtained from simple measurements. J. Chem. Educ. 70, 685–688 (1993). https://doi.org/10.1021/ed070p685
Chauhan, S., Chauhan, M.S., Jyoti, J., Rajni,: Acoustic and viscosity studies of sodium dodecyl sulfate in aqueous solutions of gelatin. J. Mol. Liq. 148, 24–28 (2009). https://doi.org/10.1016/j.molliq.2009.05.002
Chauhan, S., Pathania, L., Sharma, K., Kumar, G.: Volumetric, acoustical and viscometricbehavior of glycine and dl-alanine in aqueous furosemide solutions at different temperatures. J. Mol. Liq. 212, 656–664 (2015). https://doi.org/10.1016/j.molliq.2015.09.042
Clugston, M., Fleming, R.: Advanced Chemistry, 1st edn. Oxford University Press, Oxford (2000)
Harned, H.S., Owen, B.B.: The Physical Chemistry of Electrolyte Solutions, 3rd edn., p. 358. Reinhold, New York (1958)
Hedwig, G.R.: Thermodynamic properties of peptide solutions 3. Partial molar volumes and partial molar heat capacities of some tripeptides in aqueous solution. J. Solution Chem. 17(4), 383–397 (1988). https://doi.org/10.1007/BF00650418
Singh, H., Singh, A., Banipal, T.S., Singh, P., Bahadur, I.: Temperature and concentration dependent physicochemical interactions of L-ascorbic acid in aqueous LiCl solution: Experimental and theoretical study. Coll. Surf. A 623, 126672 (2021). https://doi.org/10.1016/j.colsurfa.2021.126672
Hepler, L.G.: Thermal expansion and structure in water and aqueous solutions. Can. J. Chem. 47(24), 4613–5461 (1969). https://doi.org/10.1139/v69-762
Naseem, B., Arif, I., Jamal, M.A.: Kosmotropic and chaotropicbehavior of hydrated ions in aqueous solutions in terms of expansibility and compressibility parameters. Arab. J. Chem. 14(11), 103405 (2021). https://doi.org/10.1016/j.arabjc.2021.103405
Hribar, B., Southall, N.T., Vlachy, V., Dill, K.A.: How ions affect the structure of water. J. Am. Chem. Soc. 124(41), 12302–12311 (2002). https://doi.org/10.1021/ja026014h
Banipal, T.S., Singh, K., Banipal, P.K., Kaur, J.: Study of interactions of l-aspartic acid and l-glutamic acid with some metal acetates through volumetric behaviour over the temperature range (288.15 to 318.15) K. J. Chem. Thermodyn. 40(7), 1166–1185 (2008). https://doi.org/10.1016/j.jct.2008.02.007
Friedman, L., Krishnan, C.V., Franks, F.: Water a Comprehensive Treatise. Plenum Press, New York (1973)
Sarkar, A., Sinha, B.: Volumetric, acoustic and transport properties of metformin hydrochloride drug in aqueous D-glucose solutions at T = (298.15–318.15) K. J. Solution Chem. 46, 424–445 (2017). https://doi.org/10.1007/s10953-017-0584-0
Ali, A., Shahjahan: Volumetric, viscometric and refractive index behavior of some α-amino acids in aqueous tetrapropylammonium bromide at different temperatures. J. Iran. Chem. Soc. 3(4), 340–350 (2006). https://doi.org/10.1007/BF03245957
McMillan, W.G., Mayer, J.E.: The statistical thermodynamics of multicomponent systems. J. Chem. Phys. 13(7), 276–305 (1945). https://doi.org/10.1063/1.1724036
Krishnan, C.V., Friedman, H.L.: Enthalpies of alkyl sulfonates in water, heavy water, and water-alcohol mixtures and the interaction of water with methylene groups. J. Solution Chem. 2(1), 37–51 (1973). https://doi.org/10.1007/BF00645870
Laidler, K.J., Meiser, J.H.: Physical Chemistry, p. 269. Benjamin/Cummings, San Francisco (1982)
Fuoss, R.M.: Conductimetric determination of thermodynamic pairing constants for symmetrical electrolytes. Proc. Natl. Acad. Sci. U.S.A. 77(1), 34–38 (1980)
Wahab, A., Mahiuddin, S.: Isentropic compressibility, electrical conductivity, shear relaxation time, surface tension, and Raman spectra of aqueous zinc nitrate solutions. J. Chem. Eng. Data 49, 126–132 (2004). https://doi.org/10.1021/je0302001
Mondal, M., Basak, S., Choudhury, S., Ghosh, N.N., Roy, M.N.: Investigation of molecular interactions insight into some biologically active amino acids and aqueous solutions of an anti-malarial drug by physicochemical and theoretical approach. J. Mol. Liq. 341, 116933 (2021). https://doi.org/10.1016/j.molliq.2021.116933
Raymond, M.: Fuoss, Conductance-concentration function for the paired ion model. J. Phys. Chem. 82(22), 7978–2427 (2018). https://doi.org/10.1021/j100511a017
Fuoss, R.M., Accascina, F.: Electrolytic Conductance. Interscience, New York (1959)
Mehrdad, A., Hajikarimi, M.: Conductometric investigation of ceftriaxone disodium in aqueous solutions of 1-propanol and 2-propanol. J. Chem. Thermodyn. 142, 105972 (2020). https://doi.org/10.1016/j.jct.2019.105972
Wright, M.R.: An Introduction to Aqueous Electrolyte Solutions. Wiley, Hoboken (2007)
Apelblat, A.: Limiting conductances of electrolytes and the Walden product in mixed solvents in a phenomenological approach. J. Phys. Chem. B 112(23), 7032–7044 (2008). https://doi.org/10.1021/jp802113v
Yan, Z., Li, W., Zhang, Q., Wang, X., Wang, J.: Effect of sodium caproate on the volumetric and conductometric properties of glycyl-l-glutamine and l-alanyl-l-glutamine in aqueous solution at 298.15 K. Fluid Phase Equil. 301, 156–162 (2011). https://doi.org/10.1016/j.fluid.2010.11.015
Borun, A., Stasiewicz, A.W.: Conductivity studies of 1:1 electrolytes in water + methanol mixtures at 298.15 K. J. Mol. Liq. 344, 117695 (2021). https://doi.org/10.1016/j.molliq.2021.117695
Yan, Z., Wen, X., Kang, Y., Zhang, S., Wu, S.: Volumetric and conductometric studies on the interactions of dipeptides with potassium perfluoroalkanesulfonate in aqueous solution at different temperatures. J. Chem. Thermodyn. 93, 172–178 (2016). https://doi.org/10.1016/j.jct.2015.10.004
Robinson, R.A., Stokes, R.H.: Electrolyte Solutions, p. 571. Butterworths, London (1965)
Jones, G., Dole, M.: The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J. Am. Chem. Soc. 51(10), 2950–2964 (1929). https://doi.org/10.1021/ja01385a012
PatilR, S., Shaikh, V.R., PatilP, D., BorseA, U., PatilK, J.: The viscosity B and D coefficients (Jones–Dole equation) studies in aqueous solutions of alkyltrimethylammonium bromides at 298.15 K. J. Mol. Liq. 200, 416–424 (2014). https://doi.org/10.1016/j.molliq.2014.11.003
Crudden, J., Delaney, G.M., Feakins, D., O’Reilly, P.J., Waghorne, W.E., Lawrence, K.G.: The viscosity and structure of solutions. Part 2.—Measurement of the B coefficient of viscosity for alkali-metal chlorides in propan-1-ol–water mixtures at 25 and 35° C. J. Chem. Soc. Faraday Trans. 1 82, 2195–2206 (1986). https://doi.org/10.1039/F19868202195
Glasstone, S., Laidle, K.J., Eyring, H.: The Theory of Rate Processes. McGraw Hill, New York (1941)
Jiang, X., Zhu, C., Ma, Y.: Volumetric and viscometric studies of amino acids in l-ascorbic acid aqueous solutions at T= (293.15 to 323.15)K. J. Chem. Thermodyn. 71, 50–63 (2014). https://doi.org/10.1016/j.jct.2013.11.002
Ren, X., Zhu, C., Ma, Y.: Volumetric and viscometric study of amino acids in aqueous sorbitol solution at different temperatures. J. Chem. Thermodyn. 93, 179–192 (2016). https://doi.org/10.1016/j.jct.2015.10.002
Acknowledgements
The authors express their heartfelt gratitude to the management of Siksha O Anusandan Deemed to be University for providing the opportunity to carry out the experimental work necessary to write this article.
Author information
Authors and Affiliations
Contributions
All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content and (c) approval of the final version.
Corresponding authors
Ethics declarations
Conflict of interest
No financial or non-financial interests are directly or indirectly related to the present work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Talukdar, M., Dey, S., Panda, A. et al. Exploring the Effect of Ammonium Acetate on Volumetric, Viscometric and Conductive Properties of l-Aspartic Acid in Aqueous Medium at Different Temperatures. J Solution Chem 53, 387–415 (2024). https://doi.org/10.1007/s10953-023-01335-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01335-7