Abstract
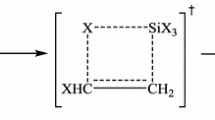
Quantum chemical calculation was performed to study the decomposition mechanism and the optimized structure of Na3AlF6 at the density functional theory (DFT). To elucidate the structural properties of the optimized Na3AlF6, the Mayer bond order (MBO) was systematically calculated. Four decomposition pathways of Na3AlF6 are determined, mainly including direct dissociation reactions and reactions with transition states. Various structure of [AlF5]2– complex ions are confirmed in the Na3AlF6 decomposition process, and trigonal bipyramidal and tetragonal pyramidal structures of [AlF5]2– complex are verified in the reactions with transition state and Al1–F2 bond-breaking reaction, respectively. The Raman shift values of the calculated main bands in the Raman spectra of Al–F complexes are in good agreement with the experimental values of the main Raman bands for molten cryolite. This result constructs a detailed decomposition scheme of Na3AlF6 and provides a theoretical basis for the investigation of its ionic structure.






Similar content being viewed by others
REFERENCES
B. J. Welch, J. Mater. 51, 24 (1999).
J. W. Evans, J. Mater. 59, 30 (2007).
V. N. Nekrasov, A. V. Suzdaltsev, O. V. Limanovskaya, et al., Electrochim. Acta 75, 296 (2012).
G. Brooks, M. Cooksey, G. Wellwood, et al., Trans. Inst. Min. Metall., Sect. C 116, 25 (2007).
S. Cikit, Z. Akdeniz, and P. A. Madden, J. Phys. Chem. B 118, 1064 (2014).
E. Robert, J. E. Olsen, V. Danek, et al., J. Phys. Chem. B 101, 9447 (1997).
M. H. Brooker, R. W. Berg, J. H. von Barner, et al., Inorg. Chem. 39, 3682 (2000).
F. Auguste, O. Tkatcheva, and H. Mediaas, et al., Inorg. Chem. 42, 6338 (2003).
X.-W. Hu, J.-Y. Qu, Z.-W. Wang, et al., Trans. Nonferr. Met. Soc. China 21, 402 (2011).
M. Lin, X. Hu, Z. Wang, et al., J. Mater. 72, 278 (2020).
E. Robert, V. Lacassagne, J.-P. Couyures, et al., Inorg. Chem. 38, 214 (1999).
V. Lacassagne, C. Bessada, D. Massiot, et al., J. Phys. Chem. B 106, 1862 (2002).
K. Machado, D. Zanghi, C. Bessada, et al., J. Phys. Chem. C 121, 10289 (2017).
B. Gilbert and T. Materne, Appl. Spectrosc. 44, 299 (1990).
E. Robert, T. Materne, and B. Gilbert, Vibr. Spectrosc. 6, 71 (1993).
M. Liška, P. Perichta, and L. T. Nagy, J. Non-Cryst. Solids 192, 309 (1995).
Z. Akdeniz, Z. Çiçek, M. P. Tosi, et al., Mod. Phys. Lett. B 12, 995 (1998).
M. J. Castiglione, M. Wilson, P. A. Madden, et al., Phys. Chem. Chem. Phys. 1, 165 (1999).
X. Lv, Z. Xu, J. Li, et al., J. Fluorine Chem. 185, 42 (2016).
X. Lv, Z. Xu, J. Li, et al., J. Mol. Struct. 1117, 105 (2016).
X. Lv, Z. Han, H. Zhang, et al., Phys. Chem. Chem. Phys. 21, 7474 (2019).
H. O. Nam, A. Bengtso, K. Vörtler, et al., J. Nucl. Mater. 449, 148 (2014).
H. Guo, J. Li, H. Zhang, et al., Chem. Phys. Lett. 730, 587 (2019).
S. Cikit, Z. Akdeniz, and P. A. Madden, J. Phys. Chem. B 118, 1064 (2014).
T. Bučko and F. Šimko, J. Chem. Phys. 144, 064502 (2016).
T. Bučko and F. Šimko, J. Chem. Phys. 148, 064501 (2018).
Y. Shao, L. Molnar, Y. Jung, et al., Phys. Chem. Chem. Phys. 8, 3172 (2006).
G. S. Picard, F. C. Bouyer, M. Leroy, et al., J. Mol. Struct.: Theochem 368, 67 (1996).
U. Groß, D. Müeller, and E. Kemnitz, Angew. Chem. Int. Ed. 42, 2626 (2003).
J.-L. You, G.-C. Jiang, H.-Y. Hou, et al., J. Raman Spectrosc. 36, 237 (2005).
V. Yu. Buz’ko, G. Yu. Chuiko, and Kh. B. Kushkhov, Russ. J. Inorg. Chem. 57, 62 (2012).
F. Xu, K. Matsumoto, and R. Hagiwara, Dalton Trans. 42, 1965 (2013).
N. Ma, J. You, L. Lu, et al., Materials 11, 1846 (2018).
R. R. Nazmutdinov, T. T. Zinkicheva, S. Yu. Vassiliev, et al., Spectrochim. Acta, Part A 75, 1244 (2010).
R. R. Nazmutdinov, T. T. Zinkicheva, S. Yu. Vassiliev, et al., Chem. Phys. 412, 22 (2013).
Z. Zhao, Z. Li, Q. Wang, et al., Res. Chem. Intermed. 41, 8471 (2015).
Y. Fu, X. Wang, X. Li, et al., AIP Adv. 6, 085305 (2016).
P.-P. Zhao, Y.-C. Wang, Y.-M. Jia, et al., Struct. Chem. 29, 1449 (2018).
Y. Fu, A. Yang, X. Wang, et al., J. Phys. D: Appl. Phys. 52, 245203 (2019).
M. J. Frisch, G. W. Trucks, D. J. Fox, et al., Gaussian 09, Revision D.01 (Gaussian, Inc., Wallingford, CT, 2009).
C. Y. Legault, CYLview, 1.0b (Univ. Sherbrooke, 2009). http://www.cylview.org
A. D. McLean and G. S. Chandler, J. Chem. Phys. 72, 5639 (1980).
R. C. Binning, Jr. and L. A. Curtiss, J. Comp. Chem. 11, 1206 (1990).
I. Mayer, Chem. Phys. Lett. 97, 270 (1983).
T. Lu and F. Chen, J. Comput. Chem. 33, 580 (2012).
S. Grimme, J. Chem. Phys. 124, 034108 (2006).
T. Schwabe and S. Grimme, Phys. Chem. Chem. Phys. 9, 3397 (2007).
A. Schäefer, H. Horn, and R. Ahlrichs, J. Chem. Phys. 97, 2571 (1992).
T. Clark, J. Chrasekhar, G. W. Spitznagel, et al., J. Comput. Chem. 4, 294 (1983).
D. Michalska and R. Wysokiński, Chem. Phys. Lett. 403, 211 (2005).
W. Humphrey, A. Dalke, and K. Schulten, J. Mol. Graph. 14, 33 (1996).
T. Lu and F. Chen, J. Mol. Graph. Modell. 38, 314 (2012).
C. Lefebvre, G. Rubez, H. Khartabil, et al., Phys. Chem. Chem. Phys. 19, 17928 (2017).
X. Hu, B. Li, J. Yu, et al., Characterization of Minerals, Metals, and Materials (Cham, Springer, 2019).
F. Bouyer, G. Picard, and J.-J. Legendre, Int. J. Quantum Chem. 61, 507 (1997).
A. J. Bridgeman, G. Cavigliasso, L. R. Ireland, et al., J. Chem. Soc., Dalton Trans. 14, 2095 (2001).
J. S. Murray and P. Politzer, Electrostatic Potentials: Chemical Applications. Encyclopedia of Computational Chemistry (West Sussex, Wiley, 1998).
J. S. Murry and P. Politzer, WIREs Comput. Mol. Sci. 1, 153 (2011).
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (grant nos. 51974081, 52004062, and 51474060), and Natural Science Foundation of Liaoning Province, China (grant no. 2019-MS-129).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhang, Y., Hu, X., Lin, M. et al. Quantum Chemical Calculation on the Decomposition Mechanism of Na3AlF6. Russ. J. Phys. Chem. 96, 1035–1043 (2022). https://doi.org/10.1134/S0036024422050302
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422050302