Abstract
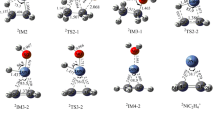
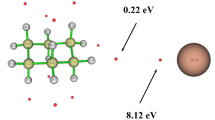
The density functional theory calculations were performed to systematically investigate the reaction of Ni+ with ethyl acetate in the gas phase. The reactive sites and reactivity were predicted by the average local ionization energy (ALIE). All possible reaction pathways were identified, which led to the formation of ketene or ethanol, two acetal units, and acetic acid or ethylene. The product distribution was discussed by means of the Curtin-Hammett principle. In addition, the properties of the chemical bonding evolution along the reaction pathway were studied using various analysis methods including atoms in molecules (AIM) and natural bond orbital (NBO). The frontier molecular orbital interactions were analyzed. The calculation results confirm that there are three reaction paths, in which the path B is the most favorable path, and acetic acid or ethylene is the main product.

ᅟ





Similar content being viewed by others
References
Hk C, Andrews L (2008). J Phys Chem A 112:12071–12080
Perera M, Metz RB, Kostko O, Ahmed M (2013). Angew Chem 125:922–925
Cox RM, Armentrout PB, Jong WA (2015). Inorg Chem 54:3584–3599
Li P, Niu WX, Gao T (2014). RSC Adv 4:29806–29817
Andrews L, Cho HG (2006). Organometallics 25:4040
Cho HG, Andrews L (2009). J Phys Chem A 113:5073–5081
Holthausen MC, Koch W (1996). J Am Chem Soc 118:9932–9940
Ma Y, Guo WY, Zhao LM, Hu SQ, Zhang J, Fu QT, Chen XF (2007). J Phys Chem A 111:6208–6216
Schroden JJ, Teo M, Davis HF (2002). J Chem Phys 117:9258
Carpenter CJ, Koppen PAM, Bowers MT (1995). J Am Chem Soc 117:10976
Chen XF, Zang H, Yeung HS, Lu XQ, Chan TWD (2012). J Mass Spectrom 47:1518–1525
Li LC, Liu JL, Shang J, Wang X, Wong NB (2007). J Theor Comput Chem 6:323–330
Zhao LM, Guo WY, Zhang RR, Wu SJ, Lu XQ (2006). ChemPhysChem 7:1345–1354
Guo WY, Yuan T, Chen XF, Zhao LM, Wu SJ (2006). J Mol Struct THEOCHEM 764:177–186
Zhao LM, Zhang RR, Guo WY, Lu XQ (2006). Chem Phys Lett 431:56–61
Sonnenfroh DM, Farrar JM (1986). J Am Chem Soc 108:3521–3522
Schroden JJ, Davis HF, Bayse CA (2007). J Phys Chem A 111:11421–11429
Zhao LM, Zhang RR, Guo WY, Wu SJ, Lu XQ (2005). Chem Phys Lett 414:28
Noll RJ, Yi SS, Weisshaar JC (1998). J Phys Chem A 102:386–394
Chen XF, Guo WY, Zhao LM, Fu QT, Ma Y (2007). J Phys Chem A 111:3566–3570
Dee SJ, Castleberry VA, Villarroel OJ, Laboren IE, Bellert DJ (2010). J Phys Chem A 114:1783–1789
Mansell A, Theis Z, Gutierrez MG, Faza ON, Lopez CS, Bellert DJ (2016). J Phys Chem A 120:2275–2284
Zhao PP, Wang YC, Sheng Y, Jia YM (2017). Comput Theor Chem 1114:140–145
Dee SJ, Castleberry VA, Villarroel OJ, Laboren IE, Frey SE, Ashley D, Bellert DJ (2009). J Phys Chem A 113:14074–14080
Castleberry VA, Dee SJ, Villarroel OJ, Laboren IE, Frey SE, Bellert DJ (2009). J Phys Chem A 113:10417–10424
Laboren IE, Villarroel OJ, Dee SJ, Castleberry VA, Klausmeyer K, Beller DJ (2011). J Phys Chem A 115:1810–1820
López CS, Faza ON, Mansell A, Bellert ZTD (2017). Organometallics 36:761–766
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, BaronenV MB, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision D.01. Gaussian Inc., Wallingford
Becke AD (1993). J Chem Phys 98:5648
Dunning TH, Peterson KA, Wilson AK (2001). J Chem Phys 114:9244
Truhlar DG (1998). Chem Phys Lett 294:45–48
Chen XF, Guo WY, Zhao LM, Fu QT (2006). Chem Phys Lett 432:27–32
La MJ, Wang YC, Wang CL, Ji DF, Jin YZ, Nian JY, Ma WP (2012). Comput Theor Chem 979:128–134
Gonzalez C, Schlegel HB (1989). J Phys Chem 90:2154
Gonzalez C, Schlegel HB (1990). J Phys Chem 94:5523
Xie M, Wang J, Bai FQ, Hao L, Zhang HX (2015). Dyes Pigments 120:74
Manzetti S, Lu T (2013). J Phys Org Chem 26:473
Bader RFW (1991). Chem Rev 91:893–928
Lu T, Chen F (2012). J Mol Graph Model 38:314
Li S, Wang YC, Wang XL, Zhang YW (2016). Comput Theor Chem 1096:74–79
Politzer P, Murray JS, Bulat FA (2010) Average local ionization energy: a review. J Mol Model 16:1731–1742
Haupert L, Poutsma JC, Wenthold PG (2009). Chem Res 42:1480–1488
Jin YZ, Wang YC, Ji DF (2013). Comput Theor Chem 1011:75–81
Shakerzadeh E (2016). Phys E 78:1–9
Cremer D, Kraka E (1984). Angew Chem Int Ed Engl 23:627–628
Santo ED, Michelini MC, Russo N (2009). Organometallics 28:3716–3726
Stalke D (2011). Chem Eur J 17:9264–9278
Wang XL, Wang YC, Li S, Zhang YW (2017). Int J Quantum Chem:e25412
Niu WX, Zhang H, Li P, Gao T (2015). Int J Quantum Chem 115:6–18
Funding
We are grateful to the financial support from the National Natural Science Foundation of China (Grant No. 21263023) and support from the Supercomputing Center of Gansu Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 135 kb)
Rights and permissions
About this article
Cite this article
Zhao, PP., Wang, YC., Jia, YM. et al. Theoretical investigation on the gas phase decomposition of ethyl acetate by Ni+. Struct Chem 29, 1449–1456 (2018). https://doi.org/10.1007/s11224-018-1125-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1125-1