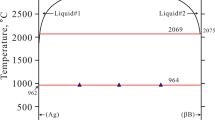
Abstract
New CALPHAD assessment of experimental data on phase equilibria and thermodynamic properties of phases in the Ag–Pd binary is performed. The results provide good description of experimental data, excepting one thermodynamic dataset which had to be excluded from calculation due to incompatibility. Results of optimization provides good description of data of phase equilibria, enthalpy of formation and activities of components obtained from 1906 to 2020 in whole range of concentrations and at temperatures from 560 to 1700 K. Differing from the results of published assessment, no artifacts (spurious miscibility gaps) were detected. The value of excess entropy for the composition Ag69.7Pd30.3 obtained from the heat capacity measured from 5 to about 560 K was not included to optimization but was used as independent test of results; the agreement seems to be well within experimental errors.





Similar content being viewed by others
REFERENCES
CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide, Ed. by N. Saunders and A. P. Miodownik (Elsevier, Amsterdam, 1998).
I. Karakaya and W. T. Thompson, Bull. Alloys Phase Diagr. 9, 237 (1988)
G. Ghosh, C. Kantner, and G. B. Olson, J. Phase Equilib. 20, 295 (1999).
C. Luef, A. Paul, H. Flandorfer, A. Kodentsov, and H. Ipser, J. Alloys Compd. 391, 67 (2005).
J. Sopousek, A. Zemanova, J. Vrestal, and P. Broz, J. Alloys Compd. 504, 431 (2010).
A. Benisek and E. Dachs, J. Alloys Compd. 527, 127 (2012).
D. Feng and P. Taskinen, J. Mater. Sci. 49, 5790 (2014).
E. M. Savitskii and N. L. Pravoverov, Russ. J. Inorg. Chem. 6, 253 (1961).
E. M. Savitskii and N. L. Pravoverov, Russ. J. Inorg. Chem. 6, 1402 (1961).
S. Hayat, A. B. Ziya, N. Ahmad, and F. Bashir, Phys. Solid State 62, 54 (2020). https://doi.org/10.1134/S1063783420010126
R. Ruer, Z. Anorg. Allg. Chem. 51, 315 (1906).
N. A. Vatolin, A. I. Timofeyev, and E. L. Dubinin, Tr. Inst. Met. Fiz. Ural Nauch. Tsentr AN SSSR 28, 236 (1971).
R. Oriani and W. K. Murphy, Acta Metall. 10, 879 (1962).
J. P. Chan and R. Hultgren, J. Chem. Thermodyn. 1, 45 (1969).
K. M. Myles, Acta Metall. 13, 109 (1965).
V. N. Eremenko, G. M. Lukashenko, and V. L. Pritula, Russ. J. Phys. Chem. 42, 346 (1968).
J. N. Pratt, Trans. Faraday Soc. 56, 975 (1960).
N. G. Schmahl, Z. Anorg. Allg. Chem. 262, 1 (1951).
N. G. Schmahl and W. Schneider, Z. Phys. Chem. 57, 218 (1968). https://doi.org/10.1524/zpch.1968.57.3_6.218
Thermo-Calc Software. http://www.thermo-calc.com. Accessed April 9, 2020.
F. Tang and B. Hallstedt, CALPHAD 55, 260 (2016).
A. Dinsdale, CALPHAD 15, 317 (1991).
J. W. Arblaster, J. Phase Equilib. Diffus. 36, 573 (2015). https://doi.org/10.1007/s11669-015-0411-5
J. W. Arblaster, Johnson Matthey Technol. Rev. 62, 48 (2018). https://doi.org/10.1595/205651318X696648
ACKNOWLEDGMENTS
Authors wish to thank Dr. A. Benisek (Universität Salzburg, Austria) for providing us with primary data of heat capacity of Ag–Pd alloy obtained in [6].
Funding
The reported study was partially funded by RFBR, project no. 19-33-90204.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pavlenko, A.S., Kabanova, E.G. & Kuznetsov, V.N. Reassessment of Ag–Pd System. Russ. J. Phys. Chem. 94, 2691–2695 (2020). https://doi.org/10.1134/S0036024420130178
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420130178