Abstract
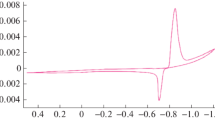
The kinetics of the electrochemical oxidation–reduction of silver in aqueous solutions of sodium nitrate was studied by potentiometry. The effect of the electrolyte concentration and temperature and sweep rate on the electrochemical behavior of silver was studied. To characterize the mechanism of silver electrooxidation, the following kinetic parameters were calculated: ion transport number (αn), diffusion coefficient (D), heterogeneous rate constant of the electrode process (Ks), and effective activation energy of the process (Eef). The data obtained may be of interest to specialists in the field of electrochemistry.




Similar content being viewed by others
REFERENCES
N. A. Kostin, V. S. Kublanovskii, and V. A. Zabludovskii, Pulse Electrolysis (Naukova Dumka, Kiev, 1989) [in Russian].
V. I. Chernenko, L. A. Snezhko, and I. I. Papanova, Coating Production by Anode-Spark Electrolysis (Khimiya, Leningrad, 1991) [in Russian].
Yu. M. Polukarov and V. V. Grinina, Itogi Nauki Tekh., Ser. Elektrokhim. 22, 62 (1985).
N. A. Kostin and O. V. Labyak, Elektrokhimiya 31, 510 (1995).
J. C. Puippe and N. Ibl, Plat. Surf. Finish. 67 (6), 68 (1980).
L. P. Shul’gin, Alternating Current Electrochemical Processes (Nauka, Leningrad, 1974) [in Russian].
I. V. Semenova, G. M. Florianovich, and A. V. Khoroshilov, Corrosion and Corrosion Protection (Fizmatlit, Moscow, 2002) [in Russian].
M. Zh. Zhurinov, A. B. Baeshov, A. K. Baeshova, and G. M. Iztleuov, Dokl. NAN RK, No. 2, 5 (2006).
G. T. Sarbaeva, A. B. Baeshov, U. A. Abdualieva, and E. Zh. Tuleshova, Vestn. NAN RK, No. 2, 73 (2017).
G. N. Zhylysbaeva, A. B. Baeshov, and B. E. Myrzabekov, Vestn. NAN RK, No. 2, 71 (2016).
G. N. Zhylysbaeva, A. B. Baeshov, R. N. Nurdillaeva, and A. N. Zhylysbaeva, Vestn. KarGU, Ser. Khim., No. 2 (78), 27 (2015).
A. K. Mamyrbekova, Russ. J. Phys. Chem. A 87, 414 (2013).
A. K. Mamyrbekova and A. K. Mamyrbekova, Russ. J. Phys. Chem. A 91, 1332 (2017).
A. B. Baeshov, E. Zh. Tuleshova, A. K. Baeshova, and U. Abdualieva, Dokl. NAN RK, No. 3, 46 (2013).
A. B. Baeshov, E. Zh. Tuleshova, A. K. Baeshova, and U. Abdualieva, Vestn. NAN RK, No. 2, 72 (2015).
E. Zh. Tuleshova, A. Bayeshov, and A. Tukibayeva, Orient. J. Chem. 29, 33 (2013).
E. Zh. Tuleshova, A. Bayeshov, A. Tukibayeva, et al., Orient. J. Chem. 31, 1867 (2015).
Z. Galus, Theoretical Foundations of Electrochemical Analysis (Panstw. Wyd. Naukowe, Warszawa, 1971) [in Polish].
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Tuleshova, E.Z. Kinetics and Mechanism of Silver Dissolution in Aqueous Sodium Nitrate. Russ. J. Phys. Chem. 92, 1820–1822 (2018). https://doi.org/10.1134/S0036024418090303
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418090303