Abstract
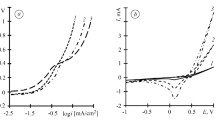
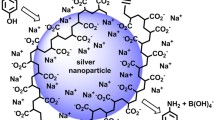
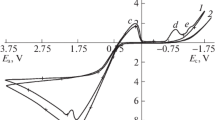
Silver sols that contain mainly small silver nanoparticles (up to 10 nm) have been synthesized by a method of electrolysis using silver electrodes under conditions of altered current polarity in the sodium polyacrylate (NaPA) solutions. It is shown that the values of the current of the anode dissolution of silver increase with increasing of NaPA concentration and the dependence of іanode—E is linear. Its proposed multistage scheme of formation stabilized by polyacrylate anion (PA−) silver nanoparticles that includes (1) ionization of silver and the complexation of produced Ag+ ions with polyacrylate, (2) cathode reduction of silver ions from the complex, (3) formation of PA− stabilized nanoclusters, and (4) aggregation of nanoclusters and formation of nanoparticles is proposed. Using UV-vis spectroscopy, observable rate constants of the nucleation and the growth of silver nanoparticles depending on temperature and sodium polyacrylate concentration have been estimated. It was found that the formation of silver nanoparticles is carried out in electrode space and is limited by diffusion. The correlations of the size of silver nanoparticles with observable rates of the nucleation and growth were shown.

"."





Similar content being viewed by others
References
Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M (2015) Silver nanoparticles as potential antibacterial agents. Molecules 20:8856–8874. https://doi.org/10.3390/molecules20058856
Anjum S, Abbasi BH, Shinwari ZK (2016) Plant-mediated green synthesis of silver nanoparticles for biomedical applications: challenges and opportunities. Pak J Bot 48:1731–1760
Srikar SK, Giri DD, Pal DB, Mishra PK, Upadhyay SN (2016) Green synthesis of silver nanoparticles: a review. Green Sustain Chem 6:34–56. https://doi.org/10.4236/gsc.2016.61004
Składanowski M, Golinska P, Rudnicka K, Dahm H, Rai M (2016) Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med Microbiol Immunol 205:603–613. https://doi.org/10.1007/s00430-016-0477-7
Alam MN, Chatterjee A, Das S, Batuta S, Mandal D, Begum NA (2015) Burmese grape fruit juice can trigger the “logic gate”-like colorimetric sensing behavior of Ag nanoparticles towards toxic metal ions. RSC Adv 5:23419–23430. https://doi.org/10.1039/c4ra16984k
Chen L, Fu X, Lu W, Chen L (2013) Highly sensitive and selective colorimetric sensing of Hg2+ based on the morphology transition of silver nanoprisms. ACS Appl Mater Interfaces 5:284–290. https://doi.org/10.1021/am3020857
Ravi SS, Christena LR, SaiSubramanian N, Anthony SP (2013) Green synthesized silver nanoparticles for selective colorimetric sensing of Hg2+ in aqueous solution at wide pH range. Analyst 138:4370–4377. https://doi.org/10.1039/c3an00320e
Li M, Gou H, Al-Ogaidi I, Wu N (2013) Nanostructured sensors for detection of heavy metals: a review. ACS Sustain Chem Eng 1:713–723. https://doi.org/10.1021/sc400019a
Zhang Z, Shen W, Xue J, Liu Y, Liu Y, Yan P, Liu J, Tang J (2018) Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res Lett 296:91–124. https://doi.org/10.1016/j.ccr.2015.03.023
Jacob JA, Kapoor S, Biswas N, Mukherjee T (2007) Size tunable synthesis of silver nanoparticles in water–ethylene glycol mixtures. Colloids Surf A Physicochem Eng Asp 301:329–334. https://doi.org/10.1016/j.colsurfa.2006.12.070
Qin Y, Ji X, Jing J, Liu H, Wu H, Yang W (2010) Size control over spherical silver nanoparticles by ascorbic acid reduction. Colloids Surf A Physicochem Eng Asp 372:172–176. https://doi.org/10.1016/j.colsurfa.2010.10.013
Kytsya AR, Bazylyak LI, Hrynda YM, Medvedevskikh YG (2014) An influence of kinetic parameters of reaction on the size of obtained nanoparticles under reduction of silver ions by hydrazine. In: Zaikov GE, Bazylak LI, Haghi AK (eds) Functional polymer blends and nanocomposites: a practical engineering approach. Apple Academic Press, New York, pp 255–262. https://doi.org/10.1201/b16895
Agnihotri S, Mukherji S, Mukherji S (2014) Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv 4:3974–3983. https://doi.org/10.1039/c3ra44507k
Ajitha B, Reddy YAK, Reddy PS (2015) Enhanced antimicrobial activity of silver nanoparticles with controlled particle size by pH variation. Powder Technol 269:110–117. https://doi.org/10.1016/j.powtec.2014.08.049
Kundu S, Dai W, Chen Y, Ma L, Yue Y, Sinyukov AM, Liang H (2017) Shape-selective catalysis and surface enhanced Raman scattering studies using Ag nanocubes, nanospheres and aggregated anisotropic nanostructures. J Colloid Interface Sci 498:248–262. https://doi.org/10.1016/j.jcis.2017.03.058
Mallakpour S, Dinari M, Talebi M (2015) A facile, efficient, and green fabrication of nanocomposites based on L-leucine containing poly (amide-imide) and PVA-modified Ag nanoparticles by ultrasonic irradiation. Colloid Polym Sci 293:1827–1833. https://doi.org/10.1007/s00396-015-3581-0
Lah NAC, Johan MR, Samykano M, Saari MM (2018) Truncated and spheroidal Ag nanoparticles: a matter of size transformation. Colloid Polym Sci 296:121–131. https://doi.org/10.1007/s00396-017-4230-6
Koval’chuk EP, Ogenko VM, Reshetnyak OV, Pereviznyk OB, Davydenko N, Marchuk IE (2010) Surface modification of silver microparticles with 4-thioaniline. Electrochim Acta 55(18):5154–5162. https://doi.org/10.1016/j.electacta.2010.04.023
Alam MN, Roy N, Mandal D, Begum NA (2013) Green chemistry for nanochemistry: exploring medicinal plants for the biogenic synthesis of metal NPs with fine-tuned properties. RSC Adv 3:11935–11956. https://doi.org/10.1039/c3ra23133
Kytsya A, Bazylyak L, Hrynda Y, Horechyy A, Medvedevdkikh Y (2015) The kinetic rate law for the autocatalytic growth of citrate-stabilized silver nanoparticles. Int J Chem Kinet 47:351–360. https://doi.org/10.1002/kin.20913
Reetz MT, Helbig W (1994) Size-selective synthesis of nanostructured transition metal clusters. J Am Chem Soc 116:1401–1402. https://doi.org/10.1021/ja00095a051
Khaydarov RA, Khaydarov RR, Gapurova O, Estrin Y, Scheper T (2009) Electrochemical method for the synthesis of silver nanoparticles. J Nanopart Res 11:1193–1200. https://doi.org/10.1007/s11051-008-9513-x
Reicha FM, Sarhan A, Abdel-Hamid MI, El-Sherbiny IM (2012) Preparation of silver nanoparticles in the presence of chitosan by electrochemical method. Carbohydr Polym 89:236–244. https://doi.org/10.1016/j.carbpol.2012.03.002
Blandón L, Vázquez MV, Benjumea DM, Ciro G (2012) Electrochemical synthesis of silver nanoparticles and their potential use as antimicrobial agent: case study on Escherichia coli. Port Electrochim Acta 30:135–144. https://doi.org/10.4152/pea.201202135
Dobre N, Petică A, Buda M, Anicăi L, Vişan T (2014) Electrochemical synthesis of silver nanoparticles in aqueous electrolytes. UPB Sci Bull Series B 76:127–136
Thuc DT, Huy TQ, Hoang LH, Tien BC, Chung PV, Thuy NT, Le AT (2016) Green synthesis of colloidal silver nanoparticles through electrochemical method and their antibacterial activity. Mater Lett 181:173–177. https://doi.org/10.1016/j.matlet.2016.06.008
Huy TQ, Thanh NTH, Thuy NT, Chung PV, Hung PN, Le AT, Hanh NTH (2017) Cytotoxicity and antiviral activity of electrochemical-synthesized silver nanoparticles against poliovirus. J Virol Methods 241:52–57. https://doi.org/10.1016/j.jviromet.2016.12.015
Zhang Z, Patel RC, Kothari R, Johnson CP, Friberg SE (2000) Stable silver clusters and nanoparticles prepared in polyacrylate and inverse micellar solutions. J Phys Chem B 104:1176–1182. https://doi.org/10.1021/jp991569t
Lopatina LI, Sergeyev VG (2010) The effects of the molecular weight and structure of poly (acrylic acid) on the formation of “blue silver”. Mosc Univ Chem Bull 65:331–334. https://doi.org/10.3103/S002713141005010X
Ni Z, Wang Z, Sun L, Li B, Zhao Y (2014) Synthesis of poly acrylic acid modified silver nanoparticles and their antimicrobial activities. Mater Sci Eng C 41:249–254. https://doi.org/10.1016/j.msec.2014.04.059
Panáček A, Prucek R, Hrbáč J, Nevečná T, Šteffková J, Zbořil R, Kvítek L (2014) Polyacrylate-assisted size control of silver nanoparticles and their catalytic activity. Chem Mater 26:1332–1339. https://doi.org/10.1021/cm400635z
Ershov BG, Henglein A (1998) Reduction of Ag+ on polyacrylate chains in aqueous solution. J Phys Chem B 102:10663–10666. https://doi.org/10.1021/jp981906i
Usman KAS, Trinidad LJP, Payawan Jr LM (2015) Spectroscopic and electronic properties of layer-by-layer assembled gamma-irradiated silver/poly (acrylic acid) nanocomposites. Adv Mater Res 1098:19–24. https://doi.org/10.4028/www.scientific.net/AMR.1098.19
Krotikova OA, Ozerin AS, Radchenko PS, Abramchuk SS, Novakov IA (2017) Aqueous phase synthesis of silver iodide nanoparticles from a polyacrylic acid–silver complex. Colloid Polym Sci 295:99–105. https://doi.org/10.1007/s00396-016-3981-9
Natsuki J, Natsuki T, Hashimoto Y (2015) A review of silver nanoparticles: synthesis methods, properties and applications. Int J Mater Sci Appl 4:325–332. https://doi.org/10.11648/j.ijmsa.20150405.17
Shervani Z, Ikushima Y, Sato M, Kawanami H, Hakuta Y, Yokoyama T, Nagase T, Kuneida H, Aramaki K (2008) Morphology and size-controlled synthesis of silver nanoparticles in aqueous surfactant polymer solutions. Colloid Polym Sci 286:403–410. https://doi.org/10.1007/s00396-007-1784-8
Financial support
Ukrainian Government Project No. 0118U000268 “Controlled electrochemical synthesis of metal nanoparticles and nanostructured materials.”
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuntyi, О.І., Kytsya, А.R., Mertsalo, I.P. et al. Electrochemical synthesis of silver nanoparticles by reversible current in solutions of sodium polyacrylate. Colloid Polym Sci 297, 689–695 (2019). https://doi.org/10.1007/s00396-019-04488-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-019-04488-4