Abstract
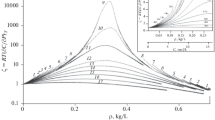
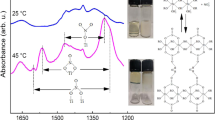
The effects of water and catalyst (formic acid) concentrations and temperature on tetraethoxysilane hydrolysis, followed by gel formation, have been studied. The catalyst concentration is found to have a stronger effect on the variation rate of dynamic viscosity of the system compared to the effect of water concentration. The thermal behavior of the thus-prepared xerogels has been studied in an air flow; distinctions have been noticed for samples whose initial dispersion-bound systems were formed at room temperature.
Similar content being viewed by others
References
M. Nocun, M. Sroda, and M. Ciecinska, Opt. Appl. 45 (1), 125 (2015).
Z.-H. Zhang, W.-Q. Wang, G.-Q. Zu, et al., Hangkong Cailiao Xuebao (J. Aeronaut. Mater.) 35 (1), 87 (2015).
O. A. Shilova, J. Sol–Gel Sci. Techn. 68, 387 (2013).
T. V. Khamova, E. S. Kolovangina, S. V. Myakin, et al., Russ. J. Gen. Chem. 83, 1594 (2013).
G. F. Krysenko, E. I. Mel’nichenko, and D. G. Epov, Russ. J. Inorg. Chem. 53, 1013 (2008).
L. Zhang and D. F. Zhang, Russ. J. Inorg. Chem. 50, 925 (2005).
J. Niedziolka-Jonsson, M. Jonsson-Niedziolka, W. Nogala, et al., Electrochim. Acta 56, 3311 (2011).
S. Cai, Q. Xue, B. Xia, et al., Mater. Lett. 156, 14 (2015).
Handbook of Sol–Gel Science and Technology. Processing, Characterization and Applications, Ed. by S. Sakka (Kluwer, Boston/Dordrecht/London/Moscow, 2005).
C. J. Brinker and G. W. Scherer, Sol–Gel Science. The Physics and Chemistry of Sol–Gel Processing (Academic Press, Boston/London/Sydney/Tokyo/Toronto/San Diego/New York, 1990).
N. A. Shabanova and P. D. Sarkisov, Fundamentals of Sol–Gel Technology of Nanosized Silica (Akademkniga, Moscow, 2004) [in Russian].
N. A. Shabanova, V. V. Popov, and P. D. Sarkisov, The Chemistry and Technology of Nanosized Oxides (Akademkniga, Moscow, 2006) [in Russian].
Y. Lu, Z.-Y. Jiang, S.-W. Xu, et al., Catal. Today 115, 263 (2006).
M. Alnaief, S. Antonyuk, C. M. Hentzschel, et al., Micropor. Mesopor. Mater 160, 167 (2012).
T. Kristiansen and K. Mathisen, J. Phys. Chem. C 118, 2439 (2014).
S. A. Lermontov, A. N. Malkova, and N. A. Sipyagina, Russ. J. Inorg. Chem. 60, 541 (2015).
S. W. Yao and H. P. Kuo, Procedia Eng. 102, 1254 (2015).
M. H. Ghasemi, S. J. Ahmadi, D. S. Moradi, et al., Rare Metals, No. 2 (2015). DOI:10.1007/s12598-0150455-z
M. Shahid, I. El Saliby, L. D. Tijing, et al., J. Nanosci. Nanotechnol. 15, 5326 (2015).
N. Lari, S. Ahangarani, and A. Shanaghi, J. Mater. Eng. Perform 24, 2645 (2015).
J. Gehring, D. Schleheck, B. Trepka, et al., ACS Appl. Mater. Interfaces 7, 1021 (2015).
S. Bernardino, N. Estrela, V. Ochoa-Mendes, et al., J. Sol–Gel Sci. Technol. 58, 545 (2011).
G. A. Kulikova, E. V. Parfenyuk, I. V. Ryabinina, et al., J. Biomed. Mater. Res. A 95A, 434 (2010).
T. V. Khamova, O. A. Shilova, G. P. Kopitsa, et al., Phys. Solid State 56, 105 (2014).
Y. Kobayashi, H. Matsudo, Y. Kubota, et al., J. Chem. Eng. Jpn 48, 112 (2015).
P. Yang, K. Matras-Postolek, X. Song, et al., Mater. Res. Bull. 70, 385 (2015).
Yu. E. Lebedeva, N. V. Popovich, L. A. Orlova, et al., Glass Ceram. 71, 400 (2015).
Yu. E. Lebedeva, N. V. Popovich, L. A. Orlova, et al., Russ. J. Appl. Chem. 87, 1625 (2014).
A. S. Chainikova, L. A. Orlova, N. V. Popovich, et al., Russ. J. Appl. Chem. 87, 1201 (2014).
Y. He, C. Chen, F. Zhong, et al., High Perform. Polym. 27, 352 (2015).
N. A. Shabanova and I. A. Belova, Glass Phys. Chem. 38, 254 (2012).
N. A. Shabanova and M. N. Sergeeva, Russ. J. Appl. Chem. 84, 1422 (2011).
L. Yang, Y. Xu, S. Qiu, and Y. Zhang, J. Polym. Res. 19 (12), 1 (2012).
S. A. Jadhav, I. Miletto, V. Brunella, et al., Polym. Adv. Technol. (in press).
S. Kim, S. Ando, and X. Wang, R. Soc. Chem. Adv. 5, 40046 (2015).
R. Suleiman, H. Dafalla, and B. El Ali, R. Soc. Chem. Adv. 5, 39155 (2015).
E. P. Simonenko, N. P. Simonenko, A. V. Derbenev, et al., Russ. J. Inorg. Chem. 58, 1143 (2013).
E. P. Simonenko, N. P. Simonenko, M. A. Zharkov, et al., J. Mater. Sci. 50, 733 (2015).
V. G. Sevastyanov, E. P. Simonenko, N. P. Simonenko, et al., Kompozity Nanostrukt. 6 (4), 198 (2014).
E. P. Simonenko and N. A. Ignatov, et al., Inorg. Mater. 46, 495 (2010).
V. G. Sevastyanov, E. P. Simonenko, N. A. Ignatov, et al., Russ. J. Inorg. Chem. 56, 661 (2011).
E. P. Simonenko, N. A. Ignatov, N. P. Simonenko, et al., Russ. J. Inorg. Chem. 56, 1681 (2011).
E. P. Simonenko, D. V. Sevast’yanov, N. P. Simonenko, et al., Russ. J. Inorg. Chem. 58, 1669 (2013).
E. P. Simonenko and A. N. Gordeev, et al., Russ. J. Inorg. Chem. 58, 1269 (2013).
E. P. Simonenko and A. N. Gordeev, et al., Russ. J. Inorg. Chem. 59, 1298 (2014).
V. G. Sevastyanov, E. P. Simonenko, A. N. Gordeev, et al., Russ. J. Inorg. Chem. 59, 1361 (2014).
V. G. Sevastyanov, E. P. Simonenko, A. N. Gordeev, et al., Russ. J. Inorg. Chem. 60, (2015).
E. P. Simonenko, N. P. Simonenko, V. G. Sevastyanov, et al., Kompozity Nanostrukt. 3 (4), 52 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.P. Simonenko, A.V. Derbenev, N.P. Simonenko, V.G. Sevastyanov, N.T. Kuznetsov, 2015, published in Zhurnal Neorganicheskoi Khimii, 2015, Vol. 60, No. 12, pp. 1579–1587.
Rights and permissions
About this article
Cite this article
Simonenko, E.P., Derbenev, A.V., Simonenko, N.P. et al. Gel formation during sol–gel synthesis of silicon dioxide. Russ. J. Inorg. Chem. 60, 1444–1451 (2015). https://doi.org/10.1134/S0036023615120220
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023615120220