Abstract
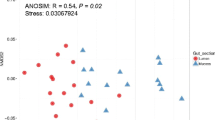
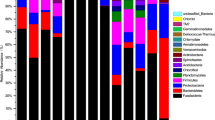
The composition of the microbiota of the digestive tract of two fish species differing in feeding habits—perch Perca fluviatilis and Prussian carp Carassius gibelio from Malye Chany Lake (West Siberia) was studied by sequencing of the V3–V4 region of the 16S ribosomal RNA gene using the Illumina MiSeq platform. During the vegetative season, the composition of the gastrointestinal microbiota of the studied species had varied with the highest diversity of bacteria in the gastrointestinal tract of perch in spring, while in the intestine of Prussian carp in spring and autumn. The perch gastrointestinal microbiota was dominated by Sediminibacterium, unclassified and uncultivated Chitinophagaceae, Sphingomonas, Caulobacter, Phyllobacterium, Haematospirillum, Cetobacterium, Vibrio, Aeromonas, and Plesiomonas; in Prussian carp, Sediminibacterium, unclassified Vibrionaceae and Chitinophagaceae, Sphingomonas, Caulobacter, Vibrio and Aeromonas. It is assumed that changes in the composition of microbial communities during the vegetative season are associated with the seasonal changes in water temperature and food resources of the lake.
Similar content being viewed by others
REFERENCES
Al-Harbi, A.H. and Uddin, M.N., Seasonal variation in the intestinal bacterial flora of hybrid tilapia (Oreochromis niloticus × Oreochromis aureus) cultured in earthen ponds in Saudi Arabia, Aquaculture, 2004, vol. 229, nos. 1–4, pp. 37–44. https://doi.org/10.1016/S0044-8486(03)00388-0
Arias, C.R., Ray, C.L., Cai, W., and Willmon, E., Fish are not alone: characterization of the gut and skin microbiomes of largemouth bass (Micropterus salmoides), bluegill (Lepomis macrochirus), and spotted gar (Lepisosteus oculatus), J. Aquacult. Fish. Fish Sci., 2019, vol. 2, no. 2, pp. 138–154. https://doi.org/10.25177/JAFFS.2.2.RA.459
Austin, B., The bacterial microflora in fish, revised, Sci. World J., 2006, vol. 6, pp. 931–945. https://doi.org/10.1100/tsw.2006.181
Bazhenov, S.V., Khrulnova, S.A., Konopleva, M.N., and Manukhov, I.V., Seasonal changes in luminescent intestinal microflora of the fish inhabiting the Bering and Okhotsk seas, FEMS Microbiol. Lett., 2019, vol. 366, no. 4, art. ID fnz040. https://doi.org/10.1093/femsle/fnz040
Bezmaternykh, D.M., Composition, structure, and quantitative characteristics of zoobenthos of the Lake Chany in 2001, Sib. Ekol. Zh., 2005, no. 2, pp. 249–254.
Bolnick, D.I., Snowberg, L.K., Hirsch, P.E., et al., Individuals’ diet diversity influences gut microbial diversity in two freshwater fish (three spine stickleback and Eurasian perch), Ecol. Lett., 2014, vol. 17, no. 8, pp. 979–987. https://doi.org/10.1111/ele.12301
Bulatov, V.I., Rotanova, I.N., and Chernykh, D.V., Landscape-ecological and cartographic analysis of lake-basin systems in the south of Western Siberia (Chany and Kulundinskoe lakes), Sib. Ekol. Zh., 2005, no. 2, pp. 175–182.
Butt, R.L. and Volkoff, H., Gut microbiota and energy homeostasis in fish, Front. Endocrinol. (Lausanne), 2019, vol. 10, no. 9, pp. 1–12. https://doi.org/10.3389/fendo.2019.00009
Caporaso, J.G., Kuczynski, J., Stombaugh, J., et al., QIIME allows analysis of high-throughput community sequencing data, Nat. Methods, 2010, vol. 7, no. 5, pp. 335–336. https://doi.org/10.1038/nmeth.f.303
Clements, K.D., Angert, E.R., Montgomery, W.L., and Choat, J.H., Intestinal microbiota in fishes: what’s known and what’s not, Mol. Ecol., 2014, vol. 23, no. 8, pp. 1891–1898. https://doi.org/10.1111/mec.12699
Dulski, T., Kozłowski, K., and Ciesielsk, S., Habitat and seasonality shape the structure of tench (Tinca tinca L.) gut microbiome, Sci. Rep., 2020a, vol. 10, no. 4460, pp. 1–11. https://doi.org/10.1038/s41598-020-61351-1
Dulski, T., Kujawa, R., Godzieba, M., and Ciesielski, S., Effect of salinity on the gut microbiome of pike fry (Esox lucius), Appl. Sci., 2020b, vol. 10, no. 7, pp. 1–17. https://doi.org/10.3390/app10072506
Edgar, R.C., Search and clustering orders of magnitude faster than BLAST, Bioinformatics, 2010, vol. 26, no. 19, pp. 2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Egerton, S., Culloty, S., Whooley, J., et al., The gut microbiota of marine fish, Front. Microbiol., 2018, vol. 9, art. ID 873. https://doi.org/10.3389/fmicb.2018.00873
Fakruddin, M. and Mannan, K., Methods for analyzing diversity of microbial communities in natural environments, Ceylon J. Sci., Biol. Sci., 2013, vol. 42, no. 1, pp. 19–33. https://doi.org/10.4038/cjsbs.v42i1.5896
Fonseca, F., Cerqueira, R., and Fuentes, J., Impact of ocean acidification on the intestinal microbiota of the marine sea bream (Sparus aurata L.), Front. Physiol., 2019, vol. 10, no. 1446, pp. 1–10. https://doi.org/10.3389/fphys.2019.01446
Ghanbari, M., Kneifel, W., and Domig, K.J., A new view of the fish gut microbiome: advances from next-generation sequencing, Aquaculture, 2015, vol. 448, pp. 464–475. https://doi.org/10.1016/j.aquaculture.2015.06.033
Givens, C.E., A fish tale: comparison of the gut microbiome of 15 fish species and the influence of diet and temperature on its composition, PhD Thesis, Athens, GA: Univ. of Georgia, 2012. http://getd.libs.uga.edu/pdfs/givens_carrie_e_201212_phd.pdf.
Han, S., Liu, Y., Zhou, Z., et al., Analysis of bacterial diversity in the intestine of grass carp (Ctenopharyngodon idellus) based on 16S rDNA gene sequences, Aquacult. Res., 2010, vol. 42, no. 1, pp. 47–56. https://doi.org/10.1111/j.1365-2109.2010.02543.x
Huang, H., Shi, P., Wang, Y., et al., Diversity of beta-propeller phytase gene in the intestinal contents of grass carp insight into the major phosphorus release from phytate in nature, Appl. Environ. Microbiol., 2009, vol. 75, no. 6, pp. 1508–1516. https://doi.org/10.1128/AEM.02188-08
Huber, I., Spanggaard, B., Appel, K.F., et al., Phylogenetic analysis and in situ identification of the intestinal microbial community of rainbow trout (Oncorhynchus mykiss, Walbaum), J. Appl. Microbiol., 2004, vol. 96, no. 1, pp. 117–132. https://doi.org/10.1046/j.1365-2672.2003.02109.x
Kanaya, G., Yadrenkina, E.N., Zuykova, E.I., et al., Contribution of organic matter sources to cyprinid fishes in the Chany Lake–Kargat River estuary, western Siberia, Mar. Freshwater Res., 2009, vol. 60, no. 6, pp. 510–518. https://doi.org/10.1071/MF08108
Kanaya, G., Solovyev, M.M., Shikano, S., et al., Application of stable isotopic analyses for fish host–parasite systems: an evaluation tool for parasite-mediated material flow in aquatic ecosystems, Aquat. Ecol., 2019, vol. 53, no. 2, pp. 217–232. https://doi.org/10.1007/s10452-019-09684-6
Kashinskaya, E.N., Belkova, N.L., Izvekova, G.I., et al., A comparative study on microbiota from the gut of Prussian carp (Carassius gibelio) and their aquatic environmental compartments, using different molecular methods, J. Appl. Microbiol., 2015, vol. 119, no. 4, pp. 948–961. https://doi.org/10.1111/jam.12904
Kashinskaya, E.N., Andree, K.B., Simonov, E.P., and Solovyev, M.M., DNA extraction protocols may influence biodiversity detected in the intestinal microbiome: a case study from wild Prussian carp, Carassius gibelio, FEMS Microbiol. Ecol., 2017, vol. 93, no. 2, pp. 1–14. https://doi.org/10.1093/femsec/fiw240
Kashinskaya, E.N., Simonov, E.P., Kabilov, M.R., et al., Diet and other environmental factors shape the bacterial communities of fish gut in an eutrophic lake, J. Appl. Microbiol., 2018, vol. 125, no. 6, pp. 1626–1641. https://doi.org/10.1111/jam.14064
Kogan, A.B., Daily diet and the intensity of intestinal filling in fishes, Vopr. Ikhtiol., 1969, vol. 9, no. 5, pp. 596–602.
Kuz’mina, V.V., Fiziologo-biokhimicheskie osnovy ekzotrofii ryb (Physiological and Biochemical Principles of Fish Exotrophy), Moscow: Nauka, 2005.
Luo, L., Chen, X., and Cai, X., Effects of Andrographis paniculata on the variation of intestinal microflora of Ctenopharyngodon idellus, J. Fish Sci. China, 2001, vol. 25, pp. 232–237.
Michel, P. and Oberdorff, T., Feeding habits of fourteen European freshwater fish species, Cybium, 1995, vol. 19, no. 1, pp. 5–46.
Miseiko, G.N. and Mikhalina, V.V., Zoobenthos of the Lake Chany (in summer 2002), Izv. Altai. Gos. Univ., 2004, no. 3, pp. 90–93.
Nayak, S.K., Role of gastrointestinal microbiota in fish, Aquacult. Res., 2010, vol. 41, no. 11, pp. 1553–1573. https://doi.org/10.1111/j.1365-2109.2010.02546.x
Neuman, C., Hatje, E., Zarkasi, K.Z., et al., The effect of diet and environmental temperature on the faecal microbiota of farmed Tasmanian Atlantic salmon (Salmo salar L.), Aquacult. Res., 2014, vol. 47, no. 2, pp. 660–672. https://doi.org/10.1111/are.12522
Okuzumi, M. and Horie, S., Studies on the bacterial flora in the intestines of various marine fish, Bull. Jpn. Soc. Sci. Fish., 1969, vol. 35, pp. 93–100.
Opredelitel’ presnovodnykh bespozvonochnykh evropeiskoi chasti SSSR (plankton i bentos) (Guide for Identification of Freshwater Invertebrates of European Part of USSR: Plankton and Bentos), Leningrad: Gidrometeoizdat, 1977.
Opredelitel’ presnovodnykh bespozvonochnykh Rossii i sopredel’nykh territorii. Tom 2. Rakoobraznye (Guide for Identification of Freshwater Invertebrates of Russia and Adjacent Territories, Vol. 2: Crustacean), St. Petersburg: Nauka, 1995.
Özdilek, Ş.Y. and Jones, R.I., The diet composition and trophic position of introduced Prussian carp Carassius gibelio (Bloch, 1782) and native fish species in a Turkish river, Turk. J. Fish. Aquat. Sci., 2014, vol. 14, pp. 769–776. https://doi.org/10.4194/1303-2712-v14_3_19
Polenogova, O.V., Kabilov, M.R., Tyurin, M.V., et al., Parasitoid envenomation alters the Galleria mellonella midgut microbiota and immunity, thereby promoting fungal infection, Sci. Rep., 2019, vol. 9, no. 1, p. 4012. https://doi.org/10.1038/s41598-019-40301-6
Price, M.N., Dehal, P.S., and Arkin, A.P., FastTree 2—Approximately maximum-likelihood trees for large alignments, PLoS One, 2010, vol. 5, no. 3, art. ID e9490. https://doi.org/10.1371/journal.pone.0009490
Quast, C., Pruesse, E., Yilmaz, P., et al., The SILVA ribosomal RNA gene database project: improved data processing and web-based tools, Nucleic Acids Res., 2013, vol. 41, pp. D590–D596. https://doi.org/10.1093/nar/gks1219
Ringø, E., Seppola, M., Berg, A., et al., Characterization of Carnobacterium divergens strain isolated from intestine of Arctic charr (Salvelinus alpinus L.), Syst. Appl. Microbiol., 2002, vol. 25, no. 1, pp. 120–129. https://doi.org/10.1078/0723-2020-00080
Ringø, E.S., Sperstad, R., Myklebust, S., et al., Characterization of the microbiota associated with intestine of Atlantic cod (Gadus morhua L.). The effect of fish meal, standard soybean meal and a bioprocessed soybean meal, Aquaculture, 2006, vol. 261, no. 3, pp. 829–841. https://doi.org/10.1016/j.aquaculture.2006.06.030
Ringø, E., Zhou, Z., Vecino, J.L.G., et al., Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquacult. Nutr., 2016, vol. 22, no. 2, pp. 219–282. https://doi.org/10.1111/anu.12346
Romero, J., Ringø, E., and Merrifield, D.L., The gut microbiota of fish, in Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics, Ringø, E. and Merrifield, D.L., Eds., Chichester: Wiley, 2014, pp. 75–100. https://doi.org/10.1002/9781118897263.ch4
Savkin, V.M., Orlova, G.A., Vasil’ev, O.F., et al., Hydrology, in Obzor ekologicheskogo sostoyaniya ozera Chany (Zapadnaya Sibir’) (Review of Ecological State of the Lake Chany (Western Siberia)), Novosibirsk: Geo, 2015, pp. 50–60.
Shivokene, Ya., Simbiontnoe pishchevarenie u gidrobiontov i nasekomykh (Symbiotic Digestion in Hydrobionts and Insects), Vilnius: Mokslas, 1989.
Schloss, P.D., Westcott, S.L., Ryabin, T., et al., Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities, Appl. Environ. Microbiol., 2009, vol. 75, no. 23, pp. 7537–7541. https://doi.org/10.1128/AEM.01541-09
Schmidt, V.T., Smith, K.F., Melvin, D.W., and Amaral-Zettler, L.A., Community assembly of a euryhaline fish microbiome during salinity acclimation, Mol. Ecol., 2015, vol. 24, no. 10, pp. 2537–2550. https://doi.org/10.1111/mec.13177
Smirnova, N.P. and Shnitnikov, A.V., Pul’siruyushchee ozera Chany (Pulsing Lake Chany), Leningrad: Nauka, 1982
Solovyev, M.M. Kashinskaya, E.N., Izvekova, G.I., et al., Feeding habits and ontogenic changes in digestive enzyme patterns in five freshwater teleosts, J. Fish Biol., 2014, vol. 85, no. 5, pp. 1–18. https://doi.org/10.1111/jfb.12489
Solovyev, M.M., Kashinskaya, E.N., Izvekova, G.I., and Glupov, V.V., pH values and activity of digestive enzymes in the gastrointestinal tract of fish in Lake Chany (West Siberia), J. Ichthyol., 2015, vol. 55, no. 2, pp. 251–258. https://doi.org/10.1134/S0032945215010208
Solovyev, M.M., Izvekova, G.I., Kashinskaya, E.N., and Gisbert, E., Dependence of pH values in the digestive tract of freshwater fishes on some abiotic and biotic factors, Hydrobiologia, 2018, vol. 807, no. 1, pp. 67–85. https://doi.org/10.1007/s10750-017-3383-0
Sugita, H., Ishida, Y., Deguchi, Y., and Kadota, H., Bacterial flora in the gastrointestine of Tilapia nilotica adapted in seawater, Bull. Jpn. Soc. Sci. Fish., 1982, vol. 48, no. 7, pp. 987–991.
Sugita, H., Iwata, J., Miyajima, C., et al., Changes in microflora of a puffer fish Fugu niphobles, with different water temperatures, Mar. Biol., 1989, vol. 101, no. 3, pp. 299–304.
Sullam, K.E. Essinger, S.D., Lozupone, C.A., et al., Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis, Mol. Ecol., 2012, vol. 21, no. 13, pp. 3363–3378. https://doi.org/10.1111/j.1365-294X.2012.05552.x
Syvokiene, J., Mickeniene, L., and Bubinas, A., Characteristics of microflora of the digestive tract of commercial fish depending on fish feeding, Ekologija, 1999, no. 4, pp. 46–54.
Tanaka, M., Kawai, S., Seikai, T., and Burke, J.S., Development of the digestive organ system in Japanese flounder in relation to metamorphosis and settlement, Mar. Freshwater Behav. Phys., 1996, vol. 28, nos. 1–2, pp. 19–31. https://doi.org/10.1080/10236249609378976
Timofeeva, M.V., Yadrenkin, A.V., and Yadrenkina, E.N., Zooplankton of the southeastern part of the lake system of the Chany Lake and its role in feeding of fish juveniles, in Ryboproduktivnost’ ozer Zapadnoi Sibiri (Fish Productivity of the Lakes of Western Siberia), Novosibirsk: Nauka, 1991, pp. 101–109.
Uchii, K., Matsui, K., Yonekura, R., et al., Genetic and physiological characterization of the intestinal bacterial microbiota of bluegill (Lepomis macrochirus) with three different feeding habits, Microbiol. Ecol., 2006, vol. 51, no. 3, pp. 277–284. https://doi.org/10.1007/s00248-006-9018-z
van Kessel, M., Dutilh, B.E., Neveling, K., et al., Pyrosequencing of 16S rRNA gene amplicons to study the microbiota in the gastrointestinal tract of carp (Cyprinus carpio L.), AMB Express, 2011, vol. 1, no. 41, pp. 1–9. https://doi.org/10.1186/2191-0855-1-41
Wolda, H., Similarity indices, sample size and diversity, Oecologia, 1981, vol. 50, pp. 296–302. https://doi.org/10.1007/BF00344966
Wong, S. and Rawls, J.F., Intestinal microbiota composition in fishes is influenced by host ecology and environment, Mol. Ecol., 2012, vol. 21, no. 13, pp. 3100–3102. https://doi.org/10.1111/j.1365-294X.2012.05552.x
Wu, S., Gao, T., Zheng, Y., et al., Microbial diversity of intestinal contents and mucus in yellow catfish (Pelteobagrus fulvidraco), Aquaculture, 2010, vol. 303, nos. 1–4, pp. 1–7. https://doi.org/10.1016/j.aquaculture.2009.12.025
Wu, S., Wang, G., Angert, E.R., et al., Composition, diversity, and origin of the bacterial community in grass carp intestine, PLoS One, 2012, vol. 7, no. 2, art. ID e30440. https://doi.org/10.1371/journal.pone.0030440
Yang, G., Bao, B., Peatman, E., et al., Analysis of the composition of the bacterial community in puffer fish Takifugu obscurus, Aquaculture, 2007, vol. 262, nos. 2–4, pp. 183–191. https://doi.org/10.1016/j.aquaculture.2006.11.031
Zarkasi, K.Z., Abell, G.C.J., Taylor, R.S., et al., Pyrosequencing-based characterization of gastrointestinal bacteria of Atlantic salmon (Salmo salar L.) within a commercial mariculture system, J. Appl. Microbiol., 2014, vol. 117, no. 1, pp. 18–27. https://doi.org/10.1111/jam.12514
Zhou, W., Chen, X., and Zhang, D., A preliminary study on the influence of different feeding stuff on intestinal microflora of grass carp (Ctenopharyngodon idellus), J. Huazhong Agric. Univ., 1998, vol. 17, no. 3, pp. 252–256.
Funding
The study was financially supported by the Russian Foundation for Basic Research, project no. 18-34-20122.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by D. Pavlov
Rights and permissions
About this article
Cite this article
Kashinskaya, E.N., Simonov, E.P., Izvekova, G.I. et al. Variability of Composition of Microbiota of Gastrointestinal Tract of Perch Perca fluviatilis and Prussian Carp Carassius gibelio During the Vegetative Season. J. Ichthyol. 61, 955–971 (2021). https://doi.org/10.1134/S0032945221060060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945221060060