Abstract—
The analysis of the literature on the microbiome composition and metabolic properties of kefir available at the RSCI and Web of Science was carried out. Kefir has been used by humans for centuries. It is a useful product of mixed lactic and alcoholic fermentation, produced using evolutionally established associative cultures, collected in an aggregated state termed kefir grains. General characterization of kefir grains from the territorial zones of different continents (Russia, Europe, Asia, and America) is provided. The methods for differentiation and identification of individual species are described, as well as their interactions within the community. The diversity of microbial composition of kefir grains depending on local cultivation conditions and storage processes is shown. The microorganisms present in kefir have a number of properties that determine their metabolism, interaction in the community, beneficial effects on human health and immune system, which is important for the prevention and control of bacterial and viral infections, especially during the COVID-19 pandemic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Under pandemic conditions, a human organism is subject to a viral attack combined with a complex of unfavorable factors affecting the normal functioning of its major systems, which impairs the balance of the intestinal microbiome and suppresses immunity. Alterations in the composition of the microbial community of the organism caused by ecological, pharmacological, and other stress factors may be ameliorated by enrichment of the GIT microbiota with introduced beneficial microorganisms (Shenderov, 2014). This finding initiated development of a new field of microbiology, studies of probiotics, living microorganisms, certain amounts of which have beneficial effects on human and animal health when introduced into the organism with food (Meier and Steuerwald, 2005; Oleskin et al., 2020). The last decades of the previous century and the beginning of the present one are characterized by active development of nutrition science dealing with nutrition of healthy humans and development of functional foodstuffs containing probiotic microflora with desired beneficial properties. Nutriceutics include biologically active compounds positively affecting human health and may contribute to prevention of certain diseases (Shenderov, 2014; Oleskin et al., 2020).
The disciplines dealing with nutrition of patients (dietology) and healthy people (nutritiology) have been known since ancient times. Old manuscripts indicate that long ago (B.C.) Egyptians, Greeks, Jews, Romans, and Arabs have used various foodstuffs to cure and prevent diseases (Farnworth, 2005). Kefir is a useful, nutritious product with unique organoleptic properties, which exerts a positive effect on human health. Unlike other fermented milk products, it does not result from metabolic activity of a single microbial species (or closely related species), but is produced by a complex, naturally developed microbial community termed kefir grains. The microbial composition of kefir grains is not stable, which affects the quality and sanitary properties of kefir (Stadie et al., 2013). However, the experimental data on the structure of kefir microbial communities and trophic interactions between the components of established consortia are equivocal and insufficient for development of a conceptual model, which could have improved our understanding on the structure of the kefir microbiome.
The goal of the present review was to collect the information on the composition of kefir grains and the interactions in the complex microbial community, which is required for development of approaches to controlling the stability of kefir grains composition and of the functional foodstuffs and pharmaceuticals with beneficial effects on human health.
Characterization of Kefir
Kefir production originates in the Caucasus and in the mountains of Mongolia or Tibet, where before 2000 B.C kefir grains were already traditionally passed from generation to generation among the tribes and were considered the source of family wealth. It got its name from the Turkish Kefir, meaning well-being or good life, because of the overall sense of health and well-being experienced by its users (Fanworth et al., 2008). This is a fermented milk product resulting from combined lactic acid and alcohol fermentation of lactose contained in milk. Kefir is obtained by inoculation of milk, a source of biologically active compounds in its own right, with kefir grains with a relatively stable and specific ratio of bacteria and yeasts. It is known that while milk assimilation by humans is 32%, fermented milk products, including kefir, are assimilated completely. The major characteristics of kefir are as follows: acidity (pH 4.6), alcohol content 0.5‒2%; its organoleptic parameters include acid taste and yeast aroma. According to the recommended standards, it should contain at least 2.8% protein, below 10% fat, and at least 0.6% lactic acid (GOST 31454-2012). Secondary components of kefir include diacetyl, acetaldehyde, and amino acids, affecting its taste (Gradova et al., 2014). The chemical composition of kefir reflects its food value. Numerous bacterial species occurring in kefir have a high probiotic potential, including their inhibitory effect on pathogenic and putrefactive microorganisms, resistance to GIT stress (low pH and bile salts), and adhesive properties resulting from the synthesis of exopolysaccharides structurally analogous to the exopolysaccharide kefiran (Enikeev, 2011; Gradova et al., 2014). Lactic acid is the strongest antiseptic agent present in kefir. In spite of the probiotic nature of kefir as such, it may be supplemented with microbial cultures in order to achieve sufficient consumption of target-oriented probiotics (Farag et al., 2020). The leaven used in kefir production consists of naturally established microbial communities termed kefir grains. According to the present-day requirements of the standards (GOST 31454-2012) a product may be named kefir if it was produced using the leaven prepared on kefir grains without addition of pure cultures of yeasts and lactic acid bacteria, with the content of lactic acid bacteria and yeasts in the product by the end of its effective life of at least 107 CFU and 104 CFU per 1 g of the product, respectively.
Kefir Grains: Structure and Functions
Kefir grains are discrete structures containing protein (4‒5%), the polysaccharide termed kefiran (9‒10%), and a complex lamellar microbiota (Leite et al., 2013: Gradova et al., 2014). They may be described as gelatinous, white or slightly yellowish irregular masses with elastic consistency, 0.3 to 3.5 cm in diameter (Figs. 1, 2). Microscopy of kefir grains revealed smooth, bumpy surfaces and a gelatinous matrix, which was overlaying the cell aggregates as a thin polysaccharide film.
The microbiota of kefir grains is represented by cocci and short and long rods, located close to elongated yeast cells. Short rods, probably Lactobacillus kefir, are located closer to the stroma surface, while long, thin, bent rods, e.g., Lactobacillus kefiranofaciens are distributed throughout the matrix volume, with their concentration increasing to the grain center. The cocci are mainly located at the surface of yeast cells, while the rods occupy the space between the yeast cells. The yeasts are most closely bound to the kefir grain stroma and are located both at the center and at the surface of the grains. The density of microbial cells is higher at the surface of the grain than in its center (Wang et al., 2012). The numbers of microorganisms at the surface and in the center of the grains depends on their relation to oxygen and on pH values. Inside the grains, pH is very low and inhibits the growth of lactococci. Due to weak adhesive properties of Lactococcus lactis, many electron microscopic studies failed to reveal them in kefir grains, although other isolation techniques showed that L. lactis was one of the dominant species in the same grains (Cheirsilp et al., 2003; Jianzhong et al., 2009).
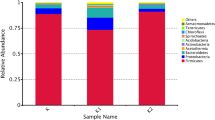
The exact microbial composition of kefir grains remains debatable. In the grain base, up to 50 bacterial and yeast species were isolated from kefirs produced in different regions (Pogačić et al., 2013). The bacterial genera most common in kefir grains from milk belong to LAB, which are responsible for 37‒90% of the microbial population (Yüksekdag et al., 2004; Miguel et al., 2010; Zanirati et al., 2015); acetic acid bacteria, yeasts, and fungi are also present (Witthuhn et al., 2005; Yang et al., 2007; Mayoa et al., 2012; Gao et al., 2012, 2013). The microbial composition of kefir grains was reported to depend significantly on their origin and local cultivation conditions (Prado et al., 2001; Kotova et al., 2016) (Table 1).
Among LAB, lactobacilli, such as Lactobacillus paracasei ssp. paracasei, L. acidophilus, L. delbrueckii ssp. bulgaricus, L. plantarum, and L. kefiranofaciens, are predominant species constituting 20% of the total LAB number (Gao et al., 2007; Wang et al., 2012; Zanirati et al., 2015). Other microorganisms present include mesophilic homofermentative lactococci Lactococcus spр. (Magalhaes et al., 2011; Garofalo et al., 2015), thermophilic Streptococcus thermophilus (Simova et al., 2002; Kok-Tas et al., 2012; Guzel-Seydium, 2015), heterofermentative lactobacilli and Leuconostoc spр., streptococci producing lactic and acetic acids, CO2, ethanol, dextran, and the substances responsible for specific aroma, such as acetoin and diacetyl (Diosma et al., 2014; Walsh et al., 2016). Acetic acid bacteria, e.g., Acetobacter fabarum, have been isolated in China (Yang et al., 2007; Gao et al., 2012; Jianzhong et al., 2009), while Acetobacter pasteurianus was found in kefirs produced in European countries (France, Belgium, Italy, and Switzerland) (Garofalo et al., 2015; Korsak et al., 2015; Kok-Tas et al., 2012). Acetic acid bacteria isolated from dairy products belong to the genus Acetobacter, comprising motile gram-negative rods, which occur singly, in pairs, or in chains. Some strains may exhibit involutionary forms: spherical, bent, filamentous, etc. They do not form spores or capsules. Ethanol is oxidized to acetic acid under oxic conditions (the so-called acetic acid fermentation); some species may oxidize acetate and lactate to CO2 and H2O. Lactose is not fermented (Montaghi et al., 1997).
Enterococci Е. durans were found in the microbiota of kefirs produced by Chinese and Turkish companies (Yang et al., 2007; Kesmen and Kacmaz, 2011). The numerous group of lactic acid bacteria of the genus Enterococcus, including Е. durans, has previously been assigned to streptococci of the serological groups D and E; during the first weeks of human life, they colonize the intestine and are a necessary culture involved in the processes of food transformation (Sycheva and Kartashova, 2015).
Unlike other fermented milk products, kefir is not the result of metabolic activity of one or several microbial species.
Kefir grains contain various species of yeasts, which ferment or do not ferment lactose, form or do not form spores (4 to 30 species, according to different sources). Those most often mentioned are Kluyveromyces marxianus, Candida kefyr, Saccharomyces cerevisiae, Saccharomyces unisporus, Torulospora delbrueckii, Pichia fermentans, and the synonyms for these species (Table 2). Predominant species, however, are Saccharomyces cerevisiae, S. unisporus, Candida kefyr, and Kluyveromyces marxianus ssp. marxianus (Fleet, 1990; Assadi, 2000; Loretan et al., 2003; Witthuhn et al., 2004, 2005; Kok-Tas et al., 2012; Diosma et al., 2014). Unlike other fermented milk products, kefir grains contain considerable amounts of yeasts (Tamang et al., 2016). The key role of yeasts in the preparation of fermented milk produce is accepted. In the course of this process, they produce the nutrients required for growth, including amino acids and vitamins, change the ambient pH, and release ethanol and CO2. The yeasts of kefir are less thoroughly studied than bacteria, although they are certainly responsible for establishment of the environment favoring growth of the kefir bacteria, as well as for production of the metabolites providing for the aroma and organoleptic properties of the product (Farnworth, 2005). Over 23 different yeast species have been isolated from kefir grains and from fermented beverages of various origin.
Differentiation and Identification of the Kefir Grains Microbiome
Initial differentiation of the microorganisms in the community includes a complex of phenotypic characteristics determined by investigation of their morphological, physiological, and biochemical properties. LAB are the most widespread bacteria in kefir and kefir grains (37 to 90% of the whole microbial population). These microbial species fall into four groups: homofermentative and heterofermentative lactic acid bacteria, and yeasts assimilating and not assimilating lactose (Gao et al., 2012). Anaerobic cultivation for the isolation of pure bacterial cultures was carried out under anoxic conditions at room temperature (21°C) on MRS agar in petri dishes. Under such conditions, bacterial colonies were formed after 3–5 days. Pure anaerobic cultures were obtained by streak inoculation. Inoculated plates were placed into anaerobic jars, in which gas packages were inserted. Complete isolation of the kefir grain components is difficult to achieve using the standard microbiological methods of plating on agar media. Morphological characteristics of the colonies of some microorganisms may be so similar that they may be mistaken for identical cultures, while the colonies with slight morphological differences may be formed by one microorganism (Sycheva and Kartashova, 2015). For example, under suboptimal unfavorable growth conditions, long-term action of physical, chemical, or biological stressors resulted in emergence of minor phenotypes (subpopulations) of lactic acid bacteria and of viable uncultured forms (Расhomov et al., 2018).
LAB are phylogenetically unrelated microorganisms of heterogeneous morphology: rods and orbs (cocci of spherical or ellipsoidal shape), which are characterized as gram-positive, not forming capsules or spores (except for the family Sporolactobacillaceae), not producing pigments (except for Leuconostoc citreum, which forms capsules and a yellow pigment), and not reducing nitrate to nitrite. They are catalase- and oxidase-negative, have no cytochromes, are aero- and acid-tolerant, nonmotile, and produce diverse amounts of lactic acid as the terminal metabolite (Lengeler et al., 2005).
The long-known members of the genera Lactococcus, Enterococcus, Lactobacillus, Leuconostoc, Pediococcus, Streptococcus, Vagococcus, Tetragenococcus, Carnobacterium, Bifodobacterium, but Lactococcus, Streptococcus, Pediococcus, Leuconostoc, Lactobacillus, and Bifodobacterium form the nucleus of this group. The genera Oenococcus, Weissella, Fructobacillus were recently added to this group (Lengeler et al., 2005; Lahtinen et al., 2012; Stoyanova, 2017). The genus Lactobacillus is morphologically highly diverse, from short coccoid cells to long, filamentous rods, 0.7‒1.1 to 3.0‒8.0 µm, occurring singly or in chains. Cell length often depends on the cultivation medium. In the case of mixed microbial populations, application of biochemical identification techniques is limited by the fact that after plating a sample of a liquid culture on solid nutrient medium, the ratio of two bacterial species may be determined only after isolation of pure cultures and investigation of all formed colonies, which is cost- and labor-consuming. The search for a more efficient approach to this problem is therefore an urgent issue. Molecular genetic identification techniques proved reliable and independent on external factors.
For identification of lactobacilli, the classical microbiological techniques (using cultural characteristics, morphology, Gram reaction, motility, presence of catalase, and the spectrum of fermented carbohydrates) are supplemented by molecular genetic techniques based on analysis of the 16S rRNA gene sequences using the MegAlign 6.00 DNASTAR Inc. software package. However, high stability of the 16S rRNA gene does not provide for unequivocal identification of closely related species. Accurate identification of the numerous species and subspecies of the phylogenetically related groups L. casei, L. plantarum, L. buchneri, and L. acidophilus is difficult, which requires a search for new genetic markers. Identification of the marker genes, analysis of which enables assessment of whole genome relationships between microorganisms is recommended for analysis of nucleotide sequences for species identification (Blaiotta et al., 2008). Analysis of the genes groEL, rplB, and rpoB revealed high polymorphism of their nucleotide sequences in members of the L. casei phylogenetic group and resulted in reliable identification of phenotypically and genetically close species within this group of lactobacilli (Shvetsov et al., 2011). The discriminative abilities of application of these genes is several times higher than that of the 16S rRNA gene. The nucleotide sequences were analyzed and combined into a common sequence using the SeqScape 2.6.0 software package (Applied Biosystems).
Among the important differentiating characteristics of yeasts is their ability to oxidize and ferment various carbohydrates, including maltose, sucrose, galactose, trehalose, etc. Yeasts grow within a relatively broad pH range (3 to 9), preferring, however, acidic media (pHopt 4.5‒5.5). Yeasts are osmophilic microorganisms; some of them survive at concentrations of sugars up to 55% and at salt concentrations up to 8%. According to their ability to assimilate lactose, several dozens of yeast strains from various taxa were subdivided into three groups: (1) utilizing lactose and capable of lactose fermentation; (2) utilizing lactose via direct oxidation; and (3) not utilizing lactose.
Apart from the classical techniques of pure culture isolation, denaturing gradient gel electrophoresis (DGGE) was used to compare the microbial profiles of kefir grains. This method is considered the most informative one for comparison of microbial communities, since investigation of microbial profiles does not require isolation of pure cultures. DGGE analysis also revealed no fundamental differences between microbial profiles of the studied kefir grain samples (Mayoa et al., 2012). According to the degree the bands were pronounced, seven microbial species were predominant. Some differences were found among the less abundant microbial groups forming difficultly discernible bands. Thus, no differences were revealed between microbial profiles of kefir grains used in various dairy plants in Russia. This was evidenced by the results of research carried out both by isolation of pure cultures with subsequent identification by 16S rRNA gene sequencing and using denaturing gradient gel electrophoresis, without isolation of pure cultures (Magalhães et al., 2011; Shevtsov et al., 2011).
A combination of all these geno- and phenotypic characteristics resulted in development of a new polyphasic method for differentiation.
High-throughput sequencing of the 16S rRNA genes with bacterial primers was carried out for molecular identification of bacterial pure cultures. DNA was isolated from the samples using the Fast DNA Spin Kit for Soil (MP Biomedicals, United States) according to the manufacturer’s instructions. The libraries of the 16S rRNA gene amplicons were obtained by PCR with universal primers to the V4 region as was described previously (Yang et al., 2007; Mayoa et al., 2012). The following primers were used: 515F (5'-GTGBCAGCMGCCGCGGTAA-3' (Stoyanova, 2017) and Pro-mod-805R (5'-GACTACNVGGGTMTCTAATCC-3' (Karaçali et al., 2018). Sequencing was carried out on MiSeq system (Illumina, United States) using the reagent kit for reading 150 nucleotides from each end. Demultiplexing and subsequent processing and analysis of the sequences were carried out using the relevant scripts of the QIIME 2 software package ver. 2019.1 The OTU table was constructed using SILVAngs (https://ngs.arb-silva.de/silvangs/).
High-throughput sequencing of yeast ITS1 region of the 18S-ITS1-5.8S-ITS2-28S rRNA complex was carried out after amplification of yeast DNA using the PCR primers ITS1F and ITS1R. After amplification the resulting products were purified using AMPure XP magnetic balls (Beckman Coulter, United States) and prepared for sequencing using the Nextera XT DNA kit (Illumina, United States) according to the manufacturer’s recommendations.
After UPARSE channels, the sequences were collected, filtered, and dereplicated. The operational taxonomic units (OTUs) were combined into groups with ≥97% sequence identity, from which chimeras were removed. The taxonomic identity was assigned using BLASTn and the Fittings v. 1-2 database. Taxonomy and OTUs were converted into a table using biom-format V1.3.1 (Fonseca et al., 2007).
The MEGAN software was used to obtain the taxonomic classification of microorganisms and to present the kefir grain diversity in accordance with theNCBI taxonomy (Zamberi et al., 2016). The position of the most abundant genus Lactobacillus (99.03%) is shown on Fig. 3. The second most abundant genus in the kefir grain was Phyllobacterium (0.11%), followed by Acinetobacter, Streptococcus, and Bacteroides in trace amounts. Diversity of the kefir grain at the species level is shown on Fig. 4; the most widespread species were Lactobacillus kefiranofaciens, followed by L. kefiri (91.66 and 2.52%, respectively).
A number of authors working with associative cultures reported the impossibility of objective determination of the structure of kefir grain communities based on the isolation of pure cultures.
Metabolic and Structural Interactions of Yeasts and Bacteria
Obtained data indicated that lactic acid bacteria of the physiological group which actively use lactose for lactic acid fermentation were probably the main producers of the system established in the kefir grains. The microorganisms belonging to another group use the products of lactose metabolism (glucose and galactose); the relations between members of this group may be either passive antagonism or cooperation. Investigation of the dynamics of lactic acid fermentation in the course of culture development revealed that the chemical transformations in the medium changed during this process. Two phases were clearly discernible in the course of carbohydrate fermentation. During the first one (the exponential growth phase), active synthesis of proteins and other cell components more reduced than hydrocarbons occurred. More oxidized products of metabolism were accumulated in the medium. The second phase was characterized by slower rates of biosynthesis and a gradual decrease in the redox potential of the culture, which resulted in accelerated proton transfer to PGA with its subsequent reduction to lactic acid. These two phases reflect redistribution of redox reactions in the course of biosynthesis of the structural elements of bacterial cells (constructive processes) and fermentation (an energy process). Close symbiotic relationships between LAB and yeasts and a stimulatory effect of yeasts of LAB growth have been demonstrated by many researchers (Motaghi et al., 1997; Aziza et al., 2012; Stoyanova et al., 2017).
Complex interactions between yeasts and bacteria, as well as their dependence on the microbial composition of kefir grains, are presently not completely understood. However, when bacteria are removed from the grain, yeasts grow less efficiently (Cheirsilp et al., 2003; Farnworth and Mainville, 2008; Rattray and O’Connel, 2011). The interaction between yeasts and LAB is of pivotal importance for a broad spectrum of fermented products, including kefir (Han et al., 2018). Both groups of microorganisms naturally support each other by various means listed below.
Lactic acid assimilation. One interesting mechanism of interaction between yeasts and LAB is implemented in the presence of lactic acid-assimilating yeasts. Lactate accumulation dames and kills LAB, even when pH is maintained by addition of alkaline solutions (Katakura et al., 2010). However, yeasts not utilizing lactose, e.g. S. cerevisiae, may use lactate as a carbon source, which results in increased pH and long-term LAB growth. Acid resistance is a physiological feature of LAB resulting from their specific energy metabolism. Acid stress causes intracellular acidification, which decreases the activity of cytoplasmic enzymes (Miyoshi et al., 2013). Transcriptome and proteome studies showed that while a number of LAB were able to enhance activity of their glycolytic enzymes under acidic, thermal, and osmotic stresses, this did not result in elevated lactate synthesis. Although research on the mechanism of diacetyl formation has been carried out for a long time, there is still no unanimous understanding concerning the biosynthesis of this compound by lactic acid bacteria. One of the pathways of diacetyl production is its synthesis from L-acetolactate, one of the intermediate products of citrate metabolism. This is an unstable compound released from bacterial cells into the medium, where it undergoes oxidative decarboxylation to diacetyl and nonoxidative decarboxylation to acetoin. Another pathway involving condensation of acetaldehyde-thiamine pyrophosphate and acetyl-CoA is considered doubtful by many authors, since the enzymes catalyzing these reactions have not been isolated. Acetate is released into the medium, and oxaloacetate is decarboxylated to form pyruvate. Diacetyl is formed in the reaction of acetyl-CoA and “active acetaldehyde,” an enzyme–oxyethylaminopyrophosphate complex. Diacetyl reduction by acetoin dehydrogenase results in acetoin formation (Fig. 5).
Such lactobacilli as Lactobacillus plantarum, L. reuteri, and L. rhamnosus and lactococci L. lactis modify pyruvate metabolism using lactate, and thus enhance the synthesis of the major energy-rich intermediates, such as ATP and NAD, EPS, and/or glycine. The level of lactate dehydrogenase (LDH), which is responsible for lactate synthesis from pyruvate, decreases significantly. Pyruvate oxidase and phosphoacetyl transferase, which are used to synthesize acetyl-CoA, are induced in Lactobacillus delbrueckii subsp. bulgaricus and L. rhamnosus under conditions of acid stress. Acetyl-CoA is redirected to biosynthesis of fatty acids, which may increase the strength and impermeability of the cell membrane (Leile et al., 2013).
CO2 production/O2 removal. CO2 may be responsible for establishment of the suitable atmosphere (decreased oxygen and increased CO2 concentrations) for growth of Lactobacillus spp. Although no works on the microorganisms isolated from kefir are available, research on other communities and microorganisms isolated from foodstuffs confirms this interaction. CO2 produced by yeasts promotes development of the characteristic acidic and yeast taste of kefir (Karaçali et al., 2018).
Supply of nutrients for bacteria. Trophic interactions and metabolite exchange (crosswise nutrition) enable survival of several groups of microorganisms under resource limitation. Yeasts were shown to promote bacterial growth by supplying vitamins, growth factors, and essential amino acids (Pahva et al., 2010; Ponomarova et al., 2017). Zygotorulaspora florentina was shown to produce essential amino acids, which support the growth of L. nagelii in mixed culture, but not in the case of cultivation as monocultures (Stadie et al., 2013).
To investigate the specifics of metabolite exchange between S. cerevisiae and two LAB groups (Lactobacillus plantarum or Lactococcus lactis), experiments were carried out with model systems using a combination of metabolic and genetic tools (Ponomarova et al., 2017). Nitrogen excess in the medium was found to favor the emergence of mutualism (a form of mutually beneficial coexistence, when the presence of a partner is a necessary condition for the existence of each of them) between yeasts and L. lactis. Interaction between L. lactis and S. cerevisiae is easily established when lactose is the major carbon source. This is another evidence of the important role of medium composition in formation of interspecific interactions.
Complex interactions between yeasts and bacteria, as well as their mutual dependencies in kefir grains, are still incompletely studied. However, when bacteria were separated from the grain, yeast growth became less efficient (Ratarura et al., 2010). Interaction of yeasts and LAB is of pivotal importance in a broad spectrum of fermented products, including kefir. Different groups of microorganisms naturally support each other by different means (Aziza et al., 2012).
Trophic interactions between microbial components were investigated using the strains isolated from kefir grains (~33 bacterial and 55 fungal isolates). Two physiological groups of lactic acid bacteria were revealed, differing in their ability to synthesize β‑galactosidase, the enzyme required for lactose fermentation. The yeast isolates were shown to possess no β‑galactosidase activity, did not utilize lactose, used glucose (actively) and galactose (at low activity), and do not form clods in milk. The approach based on assessment of the physiological activity of the isolates of lactic acid bacteria provided evidence that lactic acid bacteria of the first physiological group, possessing β-galactosidase activity, using lactose for lactic acid fermentation, and rapidly acidifying the system, were producers of the microbial community system. The presence in the system of several LAB species possessing β-galactosidase activity indicates that certain regulatory factors should control development of bacteria of this group: either competition for the substrate, or shifts of the main producers depending on conditions, such as pH changes (Cheirsilp and Radchabut, 2011). Figure 6 presents a general scheme of the trophic chain of the kefir grain associated culture including three LAB group (synthesizing and not synthesizing β-galactosidase, and bacteria with repressive β-galactosidase synthesis), as well as acetic acid bacteria and yeasts.
General scheme of the trophic chain in associative cultures of kefir grains fermenting and not fermenting lactose (from Cheirsilp and Radchabut, 2011, modified): 1, lactic acid bacteria synthesizing β-galactosidase; 2, lactic acid bacteria of group 1, in which β-galactosidase synthesis is repressed by glucose; 3, lactic acid bacteria not synthesizing β-galactosidase; 4, yeasts; and 5, acetic acid bacteria.
Depending on the medium and cultivation conditions, the microbiota of kefir grains and kefir leaving exhibits unique abilities for autoregulation. Microbial symbiosis in kefir grains provides for the preservation of kefir quality and the microbial profile of kefir grains throughout the year with only insignificant changes in the ratios of the major microbial groups. Kefir microbial composition may differ from that of kefir grains due to the differences in pH and cultivation time; this difference may also be associated with location of microorganisms within the grains. Thus, lactic acid bacteria of the genus Lactococcus are located on the surface of kefir grains, are easily desorbed into the culture liquid, and are therefore relatively numerous in kefir (Gradova et al., 2014).
Fermentation and Preservation of Kefir Grains
Increase in the biomass of a kefir grain during fermentation is the main marker for assessment of the symbiotic relationships between different microorganisms. The associative microbial culture of kefir grains is a stable, highly organized community with complex vertical and horizontal trophic relationships. The main products of fermentation of milk carbohydrates formed in the course of kefir production are lactic acid, ethanol (at a low concentration), and CO2, which are responsible for viscosity, acidity, and pungency. The secondary components, including diacetyl, acetaldehyde, and amino acids, which may also be found in kefir, are responsible for its aroma. In the course of fermentation the size and number of the grains increase; they are usually removed from fermented milk for repeated use. Their activity may be retained for many years, provided they are stored correctly (Lopitz-Otsoa et al., 2006; Garrote et al., 2010; Leite et al., 2013). Dried grains remain active for 12‒18 months, compared to 8‒10 days for moist grains. Many preservation techniques were tested, and freezing is presently considered preferable. Lyophilization of the grains has also been tested; it, however, resulted in lower lactose metabolism and in changes in the bacterial profile, compared to the original one (Farnworth et al., 2008). Kefir may be either used immediately after grain separation or stored in a refrigerator for subsequent use (Otles et al., 2003). The properties of fermented milk should be retained during storage; however, continuous metabolic activity of the residual kefir microbiota may result in changes in the composition of cooled kefir during storage (Gronnevik et al., 2011). A drastic decrease in viscosity during storage in a refrigerator at 4°C has been reported (Magra et al., 2012), while total fat, lactose, dry matter, and pH remained constant during 14 days of storage (Vieira et al., 2015), and lactic acid concentration increased slightly after 7 days of storage. While the lipolytic activity of milk fat under laboratory conditions is limited, it may still contribute to production of free fatty acids (Kim et al., 2002).
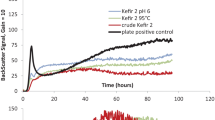
Kefir production is affected by a number of factors: raw materials, production technology, and conditions of storage of kefir and kefir grains, which should be both optimized in parallel in order to achieve the best quality of the product. Increase in temperature from 20 to 30°C resulted in elevated amounts of yeasts (from 7.1 × 106 to 107 CFU/g kefir grain and from 1.2 × 105 to 1.7 × 106 CFU/mL in the leaven) and acidic acid bacteria (from 105 to 107 CFU/g in the grains and from 4.2 × 104 to 7.0 × 106 CFU/mL in the leaven) and had an insignificant effect on the numbers of mesophilic lactic acid bacteria in kefir grains (Schoevers and Britz, 2003; Khokhlacheva et al., 2006).
However, higher fermentation temperature (25°C) resulted in a rapid pH decrease in the leaven, which inhibited growth of homo- and heterofermentative lactic acid streptococci. The leaven prepared at 25°C was shown to contain more lactic acid streptococci than in that prepared at 18–20°C. Research revealed that all strains of this species grew actively within the temperature range from 20 to 30°C, while at 35°C growth was very poor (Londero et al., 2012). All strains produced maximal biomass at 25°C. The biomass produced at 25°C was 1.3‒1.9 and 1.2‒1.8 times higher than that produced at 20 and 30°C, respectively (Khamagaeva and Vandanova, 2006). Shaking during the cultivation resulted in increased exopolysaccharide production by kefir fungal cultures and in significant differences if the qualitative and quantitative composition of the grains. Thus, shaking caused a decrease in abundance of yeasts and lactic acid bacteria in kefir grains, while the concentrations of carbohydrates and fats increased significantly (Schoevers and Britz, 2003). Screening of polysaccharide-synthesizing lactic acid bacteria revealed that out of 119 studied isolates, 60% were capable of polysaccharide synthesis. From these, 9 isolates were chosen, which synthesized polysaccharides most actively. Enhanced polysaccharide synthesis in the medium with sucrose was observed for the LAB capable of fermenting it. The cultures of Lactococcus lactis, and Leuconostoc mesenteroides were chosen as the most active exopolysaccharide producers in media with lactose and sucrose. Comparative study of kefir grain exopolysaccharides and those synthesized by monocultures, which involved IR spectroscopy and dynamic and statical light scattering revealed the similarity of EPS structure and the differences in the physicochemical properties of the polysaccharide samples with potential prebiotic activity.
Kefiran is the polysaccharide of kefir grains, which is produced by acetic acid bacteria and yeasts involved in milk fermentation. It possesses antimicrobial and wound healing activity and is able to decrease blood pressure and cholesterol level in blood serum. Kefiran in concentrations 5.9–14.3 g/L can form cryogels melting at 37°C, which may probably be applied for development of new foodstuff. Viscosity of the gels may be varied by addition of different concentrations of sucrose or fructose to kefiran solutions (Gradova et al., 2012; Zavala, 2015). When kefir grains were cultivated in milk, aeration promoted exopolysaccharide production and caused significant differences in the qualitative and quantitative composition of the grains.
Kefir grains are a complex symbiosis of several microbial species: lactic acid streptococci and lactobacilli, acetic acid bacteria, and yeasts. These grains may be used for daily kefir production at home. The popularity of kefir grains among the populace is presently steadily increasing.
The mass of kefir grains increases due to growth of microorganisms and biosynthesis of the grain components—proteins and polysaccharides. The kefir grain microbiota may be considered as a biofilm. The processes regulating biofilm formation include formation of the surface for cell attachment, intercellular interactions, and growth of a complex culture. Biofilm formation by some species has been reported, and a number of genes hypothetically responsible for adhesion or biofilm formation have been described. Biofilm formation helps the cells to survive environmental stresses, e.g., high concentrations of acid and ethanol.
Kefir contains easily digestible proteins. Minor essential acids, which are abundant in kefir, regulate the protein, carbohydrate, and lipid metabolism and have a positive effect on the regulation of human body mass, maintenance of immune response, and energy metabolism. The peptides exhibit antimicrobial and antioxidant activity in milk kefir produced by proteolysis of β-casein; 236 peptides with antimicrobial or antioxidant properties, inhibited the angiotensin converting enzyme (ACE), had immunomodulatory and antithtrombotic effects (Hamet et al., 2013; Ebner et al., 2015). Peptide F3, which was isolated from Tibetan kefir and purified, exhibited antibacterial action against Escherichia coli and Staphylococcus aureus (Miao et al., 2016). In kefir prepared from cow milk, 35 peptides were identified, which had an antihypertensive effect mediated by inhibition of the ACE activity (Amorim et al., 2019). The product is rich in such amino acids as serine, threonine, alanine, lysine, valine, isoleucine, methionine, phenylalanine, and tryptophan, which play an important role in the functioning of the central nervous system; it also contains metabolites facilitating casein digestion and assimilation by the organism (Bensmira et al., 2015).
Kefir probiotic cultures are known to regulate the immune system, promoting suppression of viral infections. The antiviral mechanism of action of kefir includes enhance macrophage production, enhanced phagocytosis, elevated production with positive differentiation of CD4+/CD8+ as a biomarker of response to treatment, of immunogloulins (IgG+ and IgA+), В-cells, Т-cells, and neutrophiles, some of which may produce antibodies if required. Kefir LAB increase the cytotoxicity of natural killer cells against tumor cells (Yamane et al., 2018). Kefir may act as an antiinflammatory agent by decreasing the expression of interleukins IL-1 and IL-6 synthesized by macrophages and T-cells and stimulating the immune response, while interferons IFN-α and type II (IFN-γ) induce the antiviral defense mechanisms. In the presence of alien antigens, elevated amounts of cytokines are produced; they act as mediators of the inflammatory process and have regulatory functions, which, in turn, induce elevated IL-6 formation, cause activation and migration of T-lymphocytes and other immune cells, resulting in the symptoms of a cytokine storm during the coronaviral infection. Thus, kefir may be an important inhibitor of the cytokine storm, which favors COVID-19 development (Nakagaki et al., 2018; Boyoglu-Barnum et al., 2019; Bornstein et al., 2020).
To conclude, deterioration of the epidemiological situation worldwide resulted in greater demand for the products and safe preparations with beneficial health effects. Traditional fermented milk produce resulting from mixed alcohol and lactic acid fermentation, including kefir, have been known since antiquity as capable of countering infections and premature aging. Milk fermentation for kefir production is a process of combined metabolism of symbiotic microbial cultures promoting formation and stability of the kefir grain microecology. Analysis of the literature data indicates that, in spite of certain differences in quantitative ratios, four microbial groups are almost always present in kefir grains: lactic acid bacteria, lactococci, acetic acid bacteria, and yeasts. What are the synergistic or antagonistic effects of these microorganisms on each other in the course of mixed-culture metabolism? Is it possible to determine one or several indicator microorganisms or indicator metabolites for quantitative assessment and evaluation of the fermentation state of kefir bacteria? Answers to these questions will not only provide the theoretical basis for investigation of kefir communities, but may be used as a guide for investigation of other microbial consortia. The contradictory data used in the development of the conceptual model, including the results of investigation of microbial composition and trophic interactions between components of the established kefir grain consortium, which are required for construction of new communities and development of approaches to control of the kefir grain stability, which may be used to create new functional food products and pharmaceuticals with beneficial effects on human health.
REFERENCES
Amorim, F.G., Coitinh, L.B., Dias, A.T., Friques, A.G.F., Monteiro, B.L., Rezende, L.C.D., Pereira, Th.M.C., Campagnaro, B.P., Pauw, E.D., Vasquez, E.C., and Quinton, L., Identification of new bioactive peptides from Kefir milk through proteopeptidomics: bioprospection of antihypertensive molecules, Food Chem., 2019, vol. 282, pp. 109–119.
Assad, M.M., Pourahmad, R., and Moazami, N., Use of isolated kefir starter cultures in kefir production, World J. Microbiol. Biotechnol., 2000, vol. 16, pp. 541–543.
Aziza, M. and Amrane, A., Diauxic growth of Geotrichum candidum and Penicillium camembertii on amino acids and glucose, Braz. J. Chem. Engin., 2012, vol. 29, pp. 203–210.
Bornstein, S.R., Rubino, F., Khunti, K., Mingrone, G., Hopkins, D., Birkenfeld, A.L., Boehm, B., Amiel, S., Holt, R.I., Skyler, J.S., DeVries, J.H., Re-nard , E., Eckel, R.H., Zimmet, P., Alberti, K.G. et al., Practical recommendations for the management of diabetes in patients with COVID-19, Lancet. Diabetes Endocrinol. Published online April 23, 2020.
Bourrie, B.C.T., Willing, B.P., and Cotter, P.D., The microbiota and health promoting characteristics of the fermented beverage kefir, Front. Microbiol., 2016, vol. 7, pp. 647–664.
Boyoglu-Barnum, S., Chirkova, T., and Anderson, L.J., Biology of infection and disease pathogenesis to guide RSV vaccine development, Front. Immunol., 2019, vol. 10, art. 1675. https://doi.org/10.3389/fimmu.2019.01675
Cheirsilp, B., Shimizu, H., and Shioya, S., Enhanced kefiran prouction by mixed culture of Lactobacillus kefiranofaciens and Saccharomyces cerevisiae, J. Biotechnol., 2003, vol. 100, pp. 43–53.
Diosma, G., Romanin, D.E., Rey-Burusco, M.F., Londero, A., and Garrote, G.L., Yeasts from kefir grains: isolation, identification, and probiotic characterization, World J. Microbiol. Biotechnol., 2014, vol. 30, pp. 43–53.
Ebner, J., Asgi Arslan, A., Fedorova, M., Hoffmann, R., Kugukgetin, A., and Pischetsrieder, M., Peptide profiling of bovine kefir reveals 236 unique peptides released from caseins during its production by starter culture or kefir grains, J. Proteomics, 2015, vol. 117, pp. 41–57.
Farag, M.A., Jomaa, S.A., and El-Wahed, A.A., The many faces of kefir fermented dairy products: quality characteristics, flavour chemistry, nutritional value, health benefits, and safety, Nutrients, 2020, vol. 12, pp. 346–359.
Farnworth, E.R. and Mainville, I., Kefir—a fermented milk product, in Handbook of Fermented Functional Foods, Farnworth, E.R., Ed., 2008, no. 2, pp. 89–127.
Farnworth, E.R., Kefir a complex probiotic, Food Science and Technology Bull.: Functional Foods, 2005, vol. 2, pp. 1–17.
Fleet, G.H., Growth of yeasts during wine fermentation, J. Wine Res., 1990, pp. 211–223.
Fonseca, G.G., Hei Latorre-García, L., del Castillo-Agudo, L., and Polaina, J., Taxonomical classification of yeasts isolated from kefir based on the sequence of their ribosomal RNA genes, World J. Microbiol. Biotechnol., 2007, vol. 23, pp. 785–791.
Gao, J., Gu, F., Abdella, N.H., Ruan, H., and He G., Investigation on culturable microflora in Tibetan kefir grains from different areas of China, J. Food Sci., 2012, vol. 77, pp. 425–433.
Gao, J., Gu, F., He, J., Xiao, J., Chen, Q., and Ruan, H., Metagenome analysis of bacterial diversity in Tibetan kefir grains, Eur. Food Res. Technol., 2013, vol. 236, pp. 549–556.
Garofalo, C., Osimani, A., Milanovič, V., Aquilanti, L., De Filippis, F., and Stellato, G., Bacteria and yeast microbiota in milk kefir grains from different Italian regions, Food Microbiol., 2015, vol. 49, pp. 123–133.
Garrote, G.L., Abraham, A.G., and De Antoni, G.L., Characteristics of kefir prepared with different grain: milk ratios, J. Dairy Res., 1998, vol. 65, pp. 149–154.
Garrote, G.L., Abraham, A.G., and De Antoni, G.L., Chemical and microbiological characterization of kefir grains, J. Dairy Res., 2001, vol. 68, pp. 639–652.
GOST (State Standard) 31454-2012: Kefir. Technical Conditions.
Gradova, N.B., Khokhlacheva, A.A., Murzina, E.D., and Myasoedova, V.V., Microbial components of kefir fungi, as a producer of kefiran exopolysaccharide, Biotechnology, 2014, no. 6, pp. 18‒26.
Guzel-Seydim, Z., Wyffels, J.T., Seydim, A.C., and Greene, A.K., Turkish kefir and kefir grains: microbial enumeration and electron microscopic observation, Int. J. Dairy Technol., 2005, vol. 58, pp. 25–29.
Hamet, M.F., Londero, A., Medrano, M., Vercammen, E., Van, H.K., and Garrote, G.L., Application of culture-dependent and culture-independent methods for the identification of Lactobacillus kefiranofaciens in microbial consortia present in kefir grains, Food Microbiol., 2013, vol. 36, pp. 327–334. https://ngs.arb-silva.de/silvangs.
Jianzhong, Z., Xiaoli, L., Hanhu, J., and Mingsheng, D., Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis, Food Microbiol., 2009, vol. 26, pp. 770–775.
Karaçali, R., Özdemir, N., and Çon, A.H., Aromatic and functional aspects of kefir produced using soya milk and Bifidobacterium species, Int. J. Dairy Technol., 2018, vol. 71, pp. 921–933.
Katakura, Y., Sano, R., Hashimoto, T., Ninomiya, K., and Shioya, S., Lactic acid bacteria display on the cell surface cytosolic proteins that recognize yeast mannan, Appl. Microbiol. Biotechnol., 2010, vol. 86, pp. 319–326.
Kesmen, Z. and Kacmaz, N., Determination of lactic microflora of kefir grains and kefir beverage by using culture-dependent and culture-independent methods, J. Food Sci., 2011, vol. 76, pp. 276–283.
Khamagayeva, I.S. and Vandanova, Ye.V., Selection of conditions for the cultivation of symbiotic starter culture for the production of kefir, Food and Processing Industry. Abstract Journal, 2006, no. 2, pp. 95‒98.
Khokhlacheva, A.A., Egorova, M.A., Kalinina, A.N., and Gradova, N.B., Trophic patterns of functioning and microbial profile of an evolutionarily established associative culture of kefir grains, Microbiology (Moscow), 2015, vol. 84, pp. 561–569.
Kim, Y.J. and Liu, R.H., Increase of conjugated linoleic acid content in milk by fermentation with lactic acid bacteria, J. Food Sci., 2002, vol. 67, pp. 1731–1737.
Kok-Tas, T., Ekinci, F.Y., and Guzel-Seydim, Z.B., Identification of microbial flora in kefir grains produced in Turkey using PCR, Int. J. Dairy Technol., 2012, vol. 65, pp. 126–131.
Korsak, N., Taminiau, B., Leclerc, M., Nezer, C., Crevecoeur, S., Ferauche, C., Detry, E., Delcenserie, V., and Daube, G., Short communication: evaluation of the microbiota of kefir samples using metagenetic analysis targeting the 16S and 26S ribosomal DNA fragments, J. Dairy Sci., 2015, vol. 98, pp. 3684–3689.
Kotova, I.B., Cherdyntseva, T.A., and Netrusov, A.I., Russian kefir grains microbial composition and its changes during production process, Adv. Exp. Med. Biol., 2016, vol. 4, pp. 93–121.
Lahtinen, S., Ouwehand, A.C., Salminen, S., and von Wright, A., Lactic Acid Bacteria: Microbiological and Functional Aspects, CRC, 2011, 4th ed.
Latorre-García, L., del Castillo-Agudo, L., and Polaina, J., Taxonomical classification of yeasts isolated from kefir based on the sequence of their ribosomal RNA genes, World J. Microbiol. Biotechnol., 2007, vol. 23, pp. 785–791.
Leite, A.M.O., Leite, D.C.A., del Aguila, E.M., Alvares, T.S., Peixoto, R.S., Miguel, M.A.I., Silva, J.T., and Paschoalin, V.M.F., Microbiological and chemical characteristics of Brazilian kefir during fermentation and storage processes, J. Dairy Sci., 2013, vol. 96, pp. 4149‒4159.
Leite, A.M.O., Miguel, M.A., Peixoto, R.S., Rosado, A.S., Silva, J.T., and Paschoalin, V.M.F., Microbiological, technological and therapeutic properties of kefir: a natural probiotic beverage, Braz. J. Microbiol., 2013, vol. 44, pp. 341–349.
Lengeler, J., Drews, G., and Schlegel, G., Eds., Biology of Prokaryotes, Wiley-Blackwell, 1999.
Londero, A., Hamet, M.F., De Antoni, G.L., Garrote, G.L., and Abraham, A.G., Kefir grains as a starter for whey fermentation at different temperatures: chemical and microbiological characterization, J. Dairy Res., 2012, vol. 79, pp. 262–271.
Lopitz-Otsoa, F., Rementeria, A., Elguezabala, N., and Garaizar, J., Kefir: a simbiotic yeasts-bacteria community with alleged healthy capabilities, Rev. Iberoam. Micol., 2006, vol. 23, pp. 67–74.
Loretan, T., Mostert, J.F., and Viljoen, B.C., Microbial flora associated with South African household kefir, South Afr. J. Sci., 2003, vol. 99, pp. 92–94.
Machado, A., Leite, D.O., Antonio, M., Miguel, L., Peixoto, R.S., Rosado, A.S., Silva, J.T., Margaret, V., and Paschoalin, F., Microbiological, technological and therapeutic properties of kefir: a natural probiotic beverage, Braz. J. Microbiol., 2013, vol. 44, pp. 341–349.
Magalhães, K.T., Pereira, G.V.M., Campos, C.R., Dragone, G., and Schwan, R.F., Brazilian kefir: structure, microbial communities and chemical composition, Braz. J. Microbiol., 2011, vol. 42, pp. 693–702.
Mainville, I., Robert, N., Lee, B., and Farnworth, E.R., Polyphasic characterization of the lactic acid bacteria in kefir, Syst. Appl. Microbiol., 2006, vol. 29, pp. 59–68.
Mayoa, B., Rachid, C.T., Peixoto, R.S., Silva, J.T., and Paschoalin, V.M., Assessment of the microbial diversity of Brazilian kefir grains by PCR-DGGE and pyrosequencing analysis, Food Microbiol., 2012, vol. 31, pp. 215–221.
Meier, R. and Steuerwald, M., Place of probiotics, Curr. Opin. Crit. Care, 2005, vol. 11, pp. 318–325.
Miao, J., Liu, G., Ke, C., Fan, W., Li, C., Chen, Y., Dixon, W., Song, M., Cao, Y., and Xiao, H., Inhibitory effects of a novel antimicrobial peptide from kefir against Escherichia coli, Food Control, 2016, vol. 65, pp. 63–72.
Miguel, M.G.C.P., Cardoso, P.G., Lago, L.A., and Schwan, R.F., Diversity of bacteria present in milk kefir grains using culture-dependent and culture-independent methods, Food Res. Int., 2010, vol. 43, pp. 1523–1528.
Mitra, S. and Ghosh, B.C., Quality characteristics of kefir as a carrier for probiotic Lactobacillus rhamnosus GG, Int. J. Dairy Technol., 2020, vol. 73, pp. 384–391.
Miyoshi, A., Rochat, T., Gratadoux, J.J., Le Loir, Y., Costa Oliveira, S., Langella, P., and Azevedo, V., Oxidative stress in Lacococcus, Gen. Mol. Res., 2003, vol. 3, pp. 348–359.
Motaghi, M., Mazaheri, M., Moazami, N., Farkhondeh, A., Fooladi, M., and Goltapeh, E., Kefir production in Iran, World J. Microbiol. Biotechnol., 1997, vol. 13, pp. 579–581.
Nakagaki, T., Nakano, Y., Yamane, T., Sakamoto, T., Nakagaki, T., and Nakano, Y., Lactic acid bacteria from kefir increase cytotoxicity of natural killer cells to tumor cells, Foods, 2018, vol. 7, p. 48.
Nalbantoglu, U., Cakar, A., Dogan, H., Abaci, N., Ustek, D., and Sayood, K., Metagenomic analysis of the microbial community in kefir grains, Food Microbiol., 2014, vol. 41, pp. 42–51.
Oleskin, A.V. and Shenderov B.A., Probiotics, prebiotics and metabiotics: problems and prospects, Physical and Rehabilitation Medicine, Medical Rehabilitation, 2020, vol. 2, no. 3, pp. 233–243.
Pahwa, S., Kaur, S., Jain, R., and Roy, N., Design based on the structure of new histidinol dehydrogenase inhibitors from Geotrichum candidum, Bioorg. Med. Chem. Lett., 2010, vol. 20, pp. 3972–3976.
Pakhomov, Y.D., Blinkova, L.P., Dmitrieva, O.V., Berdyugina, O.S., and Stoyanova, L.G., Non-culturability and nisin production of Lactococcus lactis, J. Bacteriol. Parasitol., 2013, vol. 5, pp. 2–8.
Pogačić, T., Sinko, S., Zamberlin, S., and Samarzija, D., Microbiota of kefir grains, Mljekarstvo, 2013, vol. 63, pp. 3–14.
Prado, M.R., Blandón, L.M., Vandenberghe, L.P.S., Rodrigues, C., Castro, G.R., Thomaz-Soccol, V., and Soccol, C.R., Milk kefir: composition, microbial cultures, biological activities, and related products, Front. Microbiol., 2015, vol. 6, art. 01177.
Schoevers, A. and Britz, T.J., Influence of different culturing conditions on kefir grain increase, Int. J. Dairy Techn., 2003, vol. 56, pp. 183−187.
Shenderov, B.A., Microbial ecology of man and its role in maintaining health, Metamorphoses, 2014, no. 5, pp. 72–80.
Shevtsov, A.B., Kushugulova, A.R., Tynybaeva, I.K., Kozhakhmetov, S.S., Abzhalelov, A.B., Momynaliev, K.T., and Stoyanova, L.G., Identification of phenotypically and genotypically related Lactobacillus strains based on nucleotide sequence analysis of the gro EL, rpo B, rpl B, and 16S rRNA genes, Microbiology (Moscow), 2011, vol. 80, pp. 672–681.
Simova, E., Beshkova, D., Angelov, A., Hristozova, T., Frengova, G., and Spasov, Z., Lactic acid bacteria and yeasts in kefir grains and kefir made from them, J. Ind. Microbiol. Biotechnol., 2002, vol. 28, pp. 1–6.
Stadie, J., Gulitz, A., Ehrmann, M.A., and Vogel, R.F., Metabolic activity and symbiotic interactions of lactic acid bacteria and yeasts isolated from water kefir, Food Microbiol., 2013, vol. 35, pp. 92–98.
Stoyanova, L.G., Isolation and identification of lactic acid bacteria Lactococcus lactis subsp. lactis with antimicrobial action, News of the Timiryazev Agricult. Acad., 2017, no. 5, pp. 41‒61.
Sycheva, M.V. and Kartashova, O.L., Biological properties of enterococci of various origins, J. Microbiol. Epidemiol. Immunobiol., 2015, no. 4, pp. 17‒21.
Tamang, J.P., Watanabe, K., and Holzapfel, W.H., Review: diversity of micoorganisms in global fermented foods and beverages, Front. Microbiol., 2016, vol. 7, art. 377.
Walsh, A.M., Crispie, F., Kilcawley, K., O’Sullivan, O., O’Sullivan, M.G., Claesson, M.J., and Cotter, P.D., Microbial succession and flavor production in the fermented dairy beverage kefir, mSystems, 2016, vol. 1. e00052-16, pp. 2–16.
Wang, S.Y., Chen, K.N., Lo, Y.M., Chiang, M.-L., Chen, H.-Ch., Liu, J.-R., and Chen, M.-J., Investigation of microorganisms involved in biosynthesis of the kefir grain, Food Microbiol., 2012, vol. 32, pp. 274–285.
Witthuhn, R.C., Schoeman, T., and Britz, T.J., Characterization of the microbial population at different stages of kefir production and kefir grain mass cultivation, Int. Dairy J., 2005, vol. 15, pp. 383–389.
Witthuhn, R.C., Schoeman, T., and Britz, T.J., Isolation and characterization of the microbial population of different South African kefir grains, Int. J. Dairy Technol., 2004, vol. 57, pp. 33–37.
Yamane, T., Sakamoto, T., Nakagaki, T., and Nakano, Y., Lactic acid bacteria from kefir increase cytotoxicity of natural killer cells to tumor cells, Foods, 2018, vol. 7, pp. 1–9.
Yang, X.J., Fan, M.T., Shi, J.L., and Dang, B., Isolation and identification of preponderant flora in Tibetan kefir, China Brewing, 2007, vol. 171, pp. 52–55.
Yenikeyev, R.R., Description, biosynthesis and biological effect of kefir fungi polysaccharide—kefiran, Biopharmaceutical J., 2011, vol. 3, no. 3, pp. 11−18.
Yüksekdag, Z.N., Beyatli, Y., and Aslim, B., Determination of some characteristic coccoid forms of lactic acid bacteria isolated from Turkish kefirs with natural probiotic, Food Sci. Technol., 2004, vol. 37, pp. 663–667.
Zamberi, N.R., Mohamad, N.E., Yeap, S.K., Ky, H., Beh, B.K., Liew, W.C., Tan, S.W., Ho, W.Y., Boo, S.Y., and Chua, Y.H., 16S metagenomic microbial composition analysis of kefir grain using megan and basespace, Food Biotechnol., 2016, vol. 30, pp. 219–230.
Zanirati, D.F., Abatemarco M., Jr., de Cicco Sandes, S.H., Nicoli, J.R., Cantini Nunes, A., and Neumann, E., Selection of lactic acid bacteria from Brazilian kefir grains for potential use as starter or probiotic cultures, Anaerobe, 2015, vol. 32, pp. 70–76.
Zavala, L., Gelling ability of kefiran in the presence of sucrose and fructose and physicochemical characterization of the resulting cryogels, J. Food Sci. Technol., 2015, vol. 52, pp. 5039–5047. https://ngs.arb-silva.de/silvangs.
Funding
The work was supported by the Government of the Shenzhen Shenzhen province and the MSU-BIT University, Shenzhen within the framework of the scientific project for carrying out the State Assignment MSU no. 23-1-21 (CITIS registration no. 121032300094-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies involving animals or human participants performed by any of the authors.
The authors declare that they have no conflicts of interest.
Additional information
Translated by P. Sigalevich
Abbreviations: ATP, adenosine triphosphate; CFB, coliform bacteria; DNA, deoxyribonucleic acid; GIT, gastrointestinal tract; LDH, lactate dehydrogenase; man-PTS, mannose phosphotransferase system; LAB, lactic acid bacteria; PDH, pyruvate dehydrogenase; PFL, pyruvate formate lyase; RNA, ribonucleic acid; PTS, phosphotransferase system; PGA, phosphoglyceric acid; CPM, cytoplasmic membrane; EPS, exopolysaccharides; α-ALS, α-acetolactate synthase; CD4, signals an infection or allergic process; CD8, indicates the risk of a oncological process; CD4/CD8, immunoregilatory index; IL‑1, interleukin-1, cytokine, a mediator of inflammation and immunity; IL-6, interleukine-6, induces T-cells growth and differentiation; IgА, class A immunoglubuline, a glycoprotein, indicator of humoral immunity; IgG, immunoglobulin, a blood plasma protein, antibodies as a response to infection.
Rights and permissions
About this article
Cite this article
Ding Fan, Stoyanova, L.G. & Netrusov, A.I. Microbiome and Metabiotic Properties of Kefir Grains and Kefirs Based on Them. Microbiology 91, 339–355 (2022). https://doi.org/10.1134/S0026261722100885
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722100885