Abstract
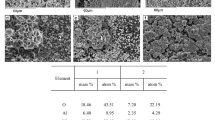
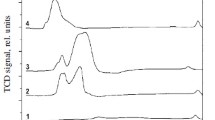
The regularities of formation of the composition and structure of a highly efficient catalyst for methanol synthesis have been revealed. The catalyst was obtained by thermal treatment of the combined CuZnAl(Cr) hydroxocarbonate followed by the reductive activation of the anion-modified zinc oxide precursor. The composition and structure of hydroxocarbonate are determined by the nature of components and their ratio. The thermal decomposition of hydroxocarbonates at 550–650 K formed the oxide catalyst; at higher temperatures, the product was an inactive mixture of oxides. The oxide catalyst is mixed zinc oxide containing the Cu2+ and Al or Cr3+ cations and residual (СО3)2– and ОН– anions, i.e., the anion-modified oxide.






Similar content being viewed by others
REFERENCES
Bems, B., Schur, M., Dassenoy, A., Junkes, H., Herein, D., and Schlögl, R., Chem. Eur. J., 2003, vol. 9, p. 2039.
Sadeghinia, M., Ghaziania, A.N.K., and Rezaei, M., Mol. Catal., 2018, vol. 456, p. 38.
Jeong, C. and Suh, Y.-W., Appl. Chem. Eng., 2016, vol. 27, no. 6, p. 555.
Güldenpfennig, A., Distaso, M., and Peukert, W., Chem. Eng. J., 2019, vol. 369, p. 996.
Jeong, Y., Kim, I., Kang, J.Y., Jeong, H., Park, J.K., Park, J.H., and Jung, J.C., J. Mol. Catal., A: Chem., 2015, vol. 400, p. 132.
Lee, W.J., Bordoloi, A., Patel, J., and Bhatelia, T., Catal. Today., 2020.
Mota, N., Guil-Lopez, R., Pawelec, B.G., Fierro, J.L.G., and Navarro, R.M., RSC Adv., 2018, vol. 8, p. 20619.
Sehested, J., J. Catal., 2019, vol. 371, p. 368.
Plyasova, L.M., Yurieva, T.M., Kriger, T.A., Makarova, O.V., Zaikovskii, V.I., Solov’eva, L.P., and Shmakov, A.N., Kinet. Katal., 1995, vol. 36, no. 3, p. 464.
Spenser, M.S., Catal. Lett., 2000, vol. 66, p. 255.
Yurieva, T.M., React. Kinet. Catal. Lett., 1995, vol. 55, no. 2, p. 513.
Baltes, C., Vukojevic, S., and Schűth, F., J. Catal., 2008, vol. 258, p. 334.
Behrens, M., J. Catal., 2009, vol. 267, p. 24.
Kondrat, S.A., Smith, P.J., Carter, J.H., Hayward, J.S., Pudge, G.J., Shaw, G.M., Spencer, M.S., Bartley, J.K., Taylor, S.H., and Hutchings, G.J., Faraday Discuss., 2017, vol. 197, p. 287.
Smith, P.J., Kondrat, S.A., Chater, P.A., Yeo, B.R., Shaw, G.M., Lu, L., Bartley, J.K., Taylor, S.H., Spencer, M.S., Kiely, C.J., Kelly, G.J., Park, C.W., and Hutchings, G.J., Chem. Sci., 2017, vol. 8, p. 2436.
Pollard, A.M., Spencer, M.S., Thomas, R.G., Williams, P.A., Holt, J., and Jennings, J.R., Appl. Catal., A, 1992, vol. 85, p. 1.
Chinchen, G.C., Denny, P.J., Jennings, J.R., Spencer, M.S., and Waugh, K.C., Appl. Catal., 1988, vol. 36, p. 1.
Waugh, K.C., Catal. Lett., 2012, vol. 142, p. 1153.
Khassin, A.A., Minyukova, T.P., and Yurieva, T.M., Mendeleev Commun., 2014, vol. 24, no. 2, p. 67.
Millar, G.J., Holm, I.H., Uwins, P.J.R., and Dennan, J., J. Chem. Soc., Faraday Trans., 1998, vol. 94, p. 593.
Behrens, M. and Schlögl, R., Z. Anorg. Allg. Chem., 2013, vol. 639, no. 15, p. 2683.
Schumann, J., Lunkenbein, T., Tarasov, A., Thomas, N., Schlögl, R., and Behrens, M., ChemCatChem, 2014, vol. 6, p. 2889.
Lunkenbein, T., Schumann, J., Behrens, M., Schlögl, R., and Willinger, M.G., Angew. Chem., Int. Ed., 2015, vol. 54, p. 4544.
Schumann, J., Tarasov, A., Thomas, N., Schlögl, R., and Behrens, M., Appl. Catal., A, 2016, vol. 516, p. 117.
Gogate, M.R., Pet. Sci. Technol., 2019, vol. 37, no. 6. P. 671.
Guil-López, R., Mota, N., Llorente, J., Millán, E., Pawelec, B., García, R., Fierro, J.L.G., and Navarro, R.M., Catal. Today., 2019. https://doi.org/10.1016/j.cattod.2019.03.034
Tarasov, A., Schumann, J., Girgsdies, F., Thomas, N., and Behrens, M., Thermochim. Acta, 2014, vol. 591, p. 1.
Roberts, A., Jambor, J., and Grice, J., Powder Diffr. J., 1986, vol. 1, p. 56.
Minyukova, T.P., Plyasova, L.M., Yurieva, T.M., Litvak, G.S., and Ketchik, S.V., Kinet. Katal., 1989, vol. 30, p. 415.
Litvak, G.S., Minyukova, T.P., Demeshkina, M.P., Plyasova, L.M., and Yurieva, T.M., React. Kinet. Catal. Lett., 1986, vol. 31, p. 403.
Pelipenko, V.V., Kochubey, D.I., Khassin, A.A., and Yurieva, T.M., React. Kinet. Catal. Lett., 2005, vol. 86, p. 307.
Khassin, A.A., Pelipenko, V.V., Minyukova, T.P., Zaikovskii, V.I., Kochubey, D.I., and Yurieva, T.M., Catal. Today., 2006, vol. 112, p. 143.
Plyasova, L.M., Yurieva, T.M., Kriger, T.A., Makarova, O.V., Zaikovskii, V.I., Solov’eva, L.P., and Shmakov, A.N., Kinet. Katal., 1995, vol. 36, p. 464.
Yurieva, T.M., Plyasova, L.M., Makarova, O.V., and Krieger, T.A., J. Mol. Catal., A: Chem., 1996, vol. 113, p. 455.
Yurieva, T.M., Plyasova, L.M., Zaikovskii, V.I., Minyukova, T.P., Bliek, A., Heuvel, J.C., Davydova, L.P., Molina, I.Yu., Demeshkina, M.P., Khassin, A.A., and Batyrev, E.D., Phys. Chem. Chem. Phys., 2004, vol. 6, p. 4522.
Hadzhieva, F.S., Anufrienko, V.F., Yurieva, T.M., Vorobiev, V.N., and Minyukova, T.P., React. Kinet. Catal. Lett., 1986, vol. 30, p. 85.
Zwiener, L., Girgsdies, F., Brennecke, D., Teschner, D., Machoke, A.G.F., Schlögl, R., and Frei, E., Appl. Catal., B, 2019, vol. 249, p. 218.
Yurieva, T.M., Plyasova, L.M., Krieger, T.A., Zaikovskii, V.I., Makarova, O.V., and Minyukova, T.P., React. Kinet. Catal. Lett., 1993, vol. 51, p. 495.
Trounov, V.A., Lebedev, V.T., Sokolov, A.E., Grushko, Yu.S., Török, Gy., Van Den Heuvel, J.C., Batyrev, É., Yurieva, T.M., and Plyasova, L.M., Crystallogr. Rep., 2007, vol. 52, no. 3, p. 474.
Clausen, B.S., Schiøts, J., Gråbæk, L., Ovesen, C.V., Jacobsen, K.W., Norskøv, J.K., and Topsøe, H., Top. Catal., 1994, vol. 1, p. 367.
Hansen, P.L., Wagner, J.B., Helveg, S., Rostrup-Nielsen, J.R., Clausen, B.S., and Topsøe, H., Science, 2002, vol. 295, p. 2053.
Guenter, M.M., Ressler, T., Berns, B., Buescher, C., Genger, T., Hinrichsen, O., Muhler, M., and Schloegl, R., Catal. Lett., 2001, vol. 71, p. 37.
Kasatkin, I., Kniep, B., and Ressler, T., Phys. Chem. Chem. Phys., 2007, vol. 7, p. 878.
Behrens, M., Studt, F., Kasatkin, I., Kühl, S., Hävecker, M., Abild-Pedersen, F., Zander, S., Girgsdies, F., Kurr, P., Kniep, B.-L., Tovar, M., Fischer, R.W., Nørskov, J.K., and Schlögl, R., Science, 2012, vol. 336, p. 893.
Wilkinson, S.K., van de Water, L.G.A., Miller, B., Simmons, M.J.H., Stitt, E.H., and Watson, M.J., J. Catal., 2016, vol. 337, p. 208.
Tisseraud, C., Comminges, C., Belin, T., Ahouari, H., Soualah, A., Pouilloux, Y., and Le Valant, A., J. Catal., 2015, vol. 330, p. 533.
Le Valant, A., Comminges, C., Tisseraud, C., Pinard, C.C.L., and Pouilloux, Y., J. Catal., 2015, vol. 324, p. 41.
Tisseraud, C., Comminges, C., Pronier, S., Pouilloux, Y., and Le Valant, A., J. Catal., 2016, vol. 343, p. 106.
Kuld, S., Thorhauge, M., Falsig, H., Elkaer, C.F., Helveg, S., Chorkendorff, I., and Sehested, J., Science, 2016, vol. 352, p. 969.
Kröhnert, J., Frei, E., Schlögl, R., and Trunschke, A., Top. Catal. 2017, vol. 60, p. 1735.
Minyukova, T.P., Khassin, A.A., and Yurieva, T.M., Kinet. Catal., 2018, vol. 59, no. 1, p. 112.
Fujitani, T. and Nakamura, J., Catal. Lett., 1998, vol. 56, p. 119.
ACKNOWLEDGMENTS
We are grateful to M.P. Demeshkina for sample preparation, to N.V. Shtertser for DTA studies, to I.Yu. Molina for XRD studies, and to Prof. L.M. Plyasova for useful discussions.
Funding
This study was performed under government contract at the Boreskov Institute of Catalysis, Siberian Branch, Russian Academy of Sciences (project no. АААА-А17-117041110045-9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Smolina
Abbreviations and notation: DTA, differential thermal analysis; XRD, X-ray diffraction analysis; EM, electron microscopy; EXAFS, extended X-ray absorption fine structure; UV–Vis, spectroscopy in the UV and visible regions; HT-CO3, high-temperature carbonates.
Rights and permissions
About this article
Cite this article
Minyukova, T.P., Khassin, A.A., Khasin, A.V. et al. Formation of Effective Copper-Based Catalysts of Methanol Synthesis. Kinet Catal 61, 886–893 (2020). https://doi.org/10.1134/S0023158420060087
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158420060087