Abstract
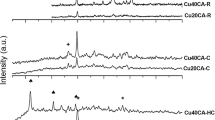
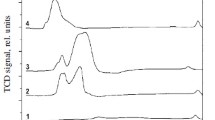
A comparative study of copper-containing catalysts with different chemical and phase compositions is performed to determine conditions for the implementation of the vapor phase and highly selective dehydrogenation of methanol to methyl formate or syngas. A thermodynamic analysis of the reaction is also performed. It is shown that Cu0 nanoparticles formed in the course of reductive activation reveal different selectivities with respect to the formation of methyl formate from methanol or its dehydrogenation with formation of syngas. By correctly selecting the catalyst composition and process conditions, high (90–100%) selectivity with respect to either methyl formate or syngas can be attained. Catalysts based on Cu–Zn hydrosilicate of the zincsilite type and on CuAlZn aurichalcite are highly selective in the process of methyl formate formation. An estimation based on experimental data shows that the productivity of Cu/SiO2 catalyst, the one most effective in dehydrogenation to syngas, is as high as 20 m3/h of syngas at a methanol vapor pressure of 1 atm, a temperature of 200°C, and a contact time of 0.5 s.
Similar content being viewed by others
References
Lee, J.S., Kim, J.C., and Kim, Y.G., Appl. Catal., A, 1990, vol. 57, no. 1, pp. 1–30.
Sodesawa, T., React. Kinet. Catal. Lett., 1986, vol. 32, no. 1, pp. 51–56.
Sodesawa, T., Nagacho, M., Onodera, A., and Nozaki, F., J. Catal., 1986, vol. 102, no. 2, pp. 460–463.
Guerreiro, E.D., Gorriz, O.F., Rivarola, L.A., and Arrúa, L.A., Appl. Catal., A, 1997, vol. 165, nos. 1–2, pp. 259–271.
Guerreiro, E.D., Gorriz, O.F., Larsen, G., and Arrúa, L.A., Appl. Catal., A, 2000, vol. 204, no. 1, pp. 33–48.
Gallo., Al., Tsoncheva, T., Marelli, M., Mihaylov, M., Dimitrov, M., Dal Santo, V., and Hadjiivanov, K., Appl. Catal., B, 2012, vol. 126, pp. 161–171.
Rodriguez-Ramos, I., Guerrero-Ruiz, A., Rojas, M.L., and Fierro, J.L.G., Appl. Catal., 1991, vol. 68, no. 1, pp. 217–228.
Chung, M.-J., Moon, D.-J., Park, K.-Y., and Ihm, S.-K., J. Catal., 1992, vol. 136, no. 2, pp. 609–612.
Lapidus, A.L., Antonyuk, S.N., Kapkin, V.D., Bruk, I.A., Sominskii, S.D., and Pechuro, N.S., Neftekhimiya, 1985, vol. 25, no. 6, pp. 761–765.
Wang, Y., Ruan, G. and Han, S., React. Kinet. Catal. Lett., 1999, vol. 67, no. 2, pp. 305–310.
Sato, S., Iijima, M., Nakayama, T., Sodesawa, T., and Nozaki, F., J. Catal., 1997, vol. 169, no. 2, pp. 447–454.
Shlegel’, L., Gutshik, D., and Rozovskii, A.Ya., Kinet. Katal., 1990, vol. 4, no. 4, p. 1000.
Gorshkov, S.V., Lin, G.I., and Rozovskii, A.Ya., Kinet. Katal., 1999, vol. 40, no. 3, p. 372.
Yurieva, T.M., Kustova, G.N., Minyukova, T.P., Poels, E.K., Bliek, A., Demeshkina, M.P., Plyasova, L.M., Krieger, T.A., and Zaikovskii, V.I., Mater. Res. Innovations, 2001, vol. 5, no. 1, pp. 3–11.
Yurieva, T.M., Plyasova, L.M., Zaikovskii, V.I., Minyukova, T.P., Bliek, A., Heuvel, J.C., Davydova, L.P., Molina, I.Yu., Demeshkina, M.P., Khassin, A.A., and Batyrev, E.D., Phys. Chem. Chem. Phys., 2004, vol. 6, no. 18, pp. 4522–4526.
Scholten, J.J.F. and Konvalinka, J.A., Trans. Faraday Soc., 1969, vol. 65, pp. 2465–2473.
Sato, S., Takahashi, R., Sodesawa, T., Yuma, K.-I., and Obata, Y., J. Catal., 2000, vol. 196, no. 1, pp. 195–199.
Evans, J.W., Wainwright, M.S., Bridgewater, A.J., and Young, D.J., Appl. Catal., 1983, vol. 7, no. 1, pp. 75–83.
Minyukova, T.P., Simentsova, I.I., Khasin, A.V., Shtertser, N.V., Baronskaya, N.A., Khassin, A.A., and Yurieva, T.M., Appl. Catal., A, 2002, vol. 237, nos. 1–2, pp. 171–180.
Yurieva, T.M., Minyukova, T.P., Kustova, G.N., Plyasova, L.M., Krieger, T.A., Demeshkina, M.P., Zaikovskii, V.I., Malakhov, V.V., and Dovlitova, L.S., Mater. Res. Innovations, 2001, vol. 5, no. 2, pp. 74–80.
Minyukova, T.P., Shtertser, N.V., Khassin, A.A., Plyasova, L.M., Kustova, G.N., Zaikovskii, V.I., Shvedenkov, Yu.G., Baronskaya, N.A., van den Heuvel, J.C., Kuznetsova, A.V., Davydova, L.P., and Yurieva, T.M., Kinet. Catal., 2008, vol. 49, no. 6, pp. 821–830.
Makarova, O.V., Yurieva, T.M., Kustova, G.N., Ziborov, A.V., Plyasova, L.M., Minyukova, T.P., and Zaikovskii, V.I., Kinet. Catal., 1993, vol. 34, no. 4, pp. 608–612.
Khassin, A.A., Kustova, G.N., Jobic, H., Yurieva, T.M., Chesalov, Yu.A., Filonenko, G.A., Plyasova, L.M., and Parmon, V.N., Phys. Chem. Chem. Phys., 2009, vol. 11, no. 29, pp. 6090–6097.
Yurieva, T.M., Catal. Today, 1999, vol. 51, nos. 3–4, pp. 457–467.
Vitanen, M.M., Jansen, W.P.A., van Welzenis, R.G., Brongerma, H.H., Brands, D.S., Poels, E.K., and Bliek, A., J. Phys. Chem. B, 1999, vol. 103, no. 29, pp. 6025–6029.
Zhang, Z., Patterson, M., Ren, M., Wang, Y., Flake, J.C., Sprunger, P.T., and Kurtz, R.L., J. Vac. Sci. Technol., A, 2013, vol. 31, no. 1, p. 01A144.
Stull, D.R., Westrum, E.F., and Sinke, G.C., The Chemical Thermodynamics of Organic Compounds, New-York: John Wiley and Sons, 1969.
US Patent 5194675, 1993.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.P. Minyukova, A.V. Khasin, A.A. Khassin, N.V. Shtertser, I.I. Simentsova, T.M. Yurieva, 2016, published in Kataliz v Promyshlennosti.
Rights and permissions
About this article
Cite this article
Minyukova, T.P., Khasin, A.V., Khassin, A.A. et al. Dehydrogenation of methanol over copper-containing catalysts. Catal. Ind. 8, 293–299 (2016). https://doi.org/10.1134/S2070050416040073
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070050416040073