Abstract
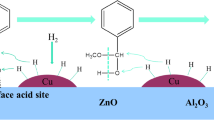
The samples of 20% Cu/γ-Al2O3, 20% Co/γ-Al2O3 and 20% Ni/γ-Al2O3 were prepared as hydrogenation catalysts for continuous synthesis of 2-(2'-hydroxy-5'-methylphenyl)benzotriazole. The best yield (86.62%) was afforded by the 20% Cu/γ-Al2O3 catalyst. The characterizations results obtained for the 20% Cu/γ-Al2O3 sample confirmed that Cu particles are evenly distributed over the surface of γ-Al2O3. A reduction of acid sites in the catalyst favored the selectivity to 2-(2'-hydroxy-5'-methylphenyl)benzotriazole. Furthermore, the effect of Cu content, reaction temperature, hydrogen pressure and liquid hourly space velocity was studied. Finally, 2-(2'-hydroxy-5'-methylphenyl)benzotriazole in 86.62% yield was attained under the optimized conditions.




Similar content being viewed by others
REFERENCES
Catalán, J., de Paz, J.L.G., Torres, R.M., and Tornero, J.D., J. Chem. Soc., Faraday Trans., 1997, vol. 93, p. 1691.
Crawford, J.C., Prog. Polym. Sci., 1999, vol. 24, p. 7.
Jpn. Patent 10 045 728, 1998.
WO Patent 2 007 036 948, 2007.
Jpn. Patent 2 004 099 448, 2004.
Gostikin, V.P., Lefedova, O.V., Marochko, A.V., and Nemtseva, M.P., Kinet. Catal., 1998, vol. 39, p. 690.
Koutsimpelis, A.G., Screttas, C.G., and Igglessi-Markopoulou, O., Heterocycles, 2005, vol. 65, p. 1393.
Jin, J., Wen, Z., Long, J., Wang, Y. M., Matsuura, T., and Meng, J.B., Synth. Commun., 2000, vol. 30, p. 829.
Farkas, R., Torincsi, M., Kolonits, P., Fekete, J., Alonso, O.J., and Novak, L., Cent. Eur. J. Chem., 2010, vol. 8, p. 300.
Whitmore, W.F., and Revukas, A.J., J. Am. Chem. Soc., 1940, vol. 62, p. 1687.
Rosevear, J., and Wilshire, J.F.K., Aust. J. Chem., 1982, vol. 35, p. 2089.
Lefedova, O.V., and Nemtseva, M.P., Russ. J. Appl. Chem., 2012, vol. 85, p. 1128.
Zuenko, M.A., Nemtseva, M.P., Lefedova, O.V., and Gladkova, E.V., Russ. J. Phys. Chem., 2006, vol. 80, p. 164.
Lefedova, O.V., Nikolaev, V.N., and Nemtseva, M.P., Russ. J. Phys. Chem., 2003, vol. 77, p. 1059.
US Patent 3230194, 1966.
Fr. Patent 2292708, 1976.
Baik, W., Park, T.H., Kim, B.H., and Jun, Y.M., J. Org. Chem., 1995, vol. 60, p. 5683.
FRG Patent 3 731 860, 1988.
US Patent 3 018 269, 1962.
CA Patent 1 154 779, 1983.
EP Patent 380 839, 1990.
FRG Patent 2 620 970, 1976.
FRG Patent 2 455 155, 1975.
Lefedova, O.V., Gostikin, V.P., and Ulitin, M.V., Russ. J. Phys. Chem., 2001, vol. 75, p. 1433.
Lefedova, O.V., Gostikin, V.P., and Nemtseva, M.P., Russ. J. Phys. Chem., 2001, vol. 75, p. 62.
Nemtseva, M.P. and Lefedova, O.V., Kinet. Catal., 1994, vol. 35, p. 547.
Wang, B.W., Fang, W.W., Si, L.L., Li, Y., Yan, X.L., Chen, L.G., and Wang, S.T., ACS Sustainable Chem. Eng., 2015, vol. 3, p. 1890.
Nemtseva, M.P., Lefedova, O.V., Gostikin, V.P., and Zuenko, M.A., Russ. J. Phys. Chem., 2003, vol. 77, p. 917.
Wang, B.W., Si, L.L., Yuan, Y.Y., Li, Y., Chen, L.G., and Yan, X.L., RSC Adv., 2016, vol. 6, p. 16766.
Si, L.L., Wang, B.W., Chen, S.P., Hou, J.R., Yan, X.L., Li, Y., and Chen, L.G., Catal. Commun., 2017, vol. 90, p. 35.
Kong, X.J. and Chen, L.G., Catal. Commun., 2014, vol. 57, p. 45.
Kong, X.J., Lai, W.C., Tian, J., Li, Y., Yan, X.L., and Chen, L., ChemCatChem., 2013, vol. 5, p. 2009.
Kong, X.J. and Chen, L.G., Appl. Catal., A, 2014, vol. 476, p. 34.
Mierczynski, P., Ciesielski, R., Zakrzewski, M., Dawid, B., Mosinska, M., Kedziora, A., Maniukiewicz, W., Dubkov, S., Gromov, D., Szynkowska, M., Witonska, I., Shtyka, O., and Maniecki, T., Kinet. Catal., 2018, vol. 59, p. 509.
Srivastava, S., Jadeja, G.C., and Parikh, J., J. Mol. Catal. A: Chem., 2017, vol. 426, p. 244.
Artyukha, E.A., Nuzhdin, A.L., Bukhtiyarova, G.A., Derevyannikova, E.A., Gerasimov, E.Y., Gladkii, A.Y., and Bukhtiyarov, V.I., Kinet. Catal., 2018, vol. 59, p. 593.
Guo, H.T., Gao, R.X., Sun, M.M., Guo, H., Wang, B.W., and Chen, L.G., ChemSusChem, 2019, vol. 12, p. 487.
Kumar, P.S., Korving, L., Keesman, K.J., van Loosdrecht, M.C.M., and Witkamp, G.J., Chem. Eng. J., 2019, vol. 358, p. 160.
Markov, P.V., Mashkovsky, I.S., Bragina, G.O., Warna, J., Gerasimov, E.Y., Bukhtiyarov, V.I., Stakheev, A.Y., and Murzin, D.Y., Chem. Eng. J., 2019, vol. 358, p. 520.
Wang, B.W., Si, L.L., Geng, J.Y., Su, Y.H., Li, Y., Yan, X.L., and Chen, L.G., Appl. Catal., B, 2017, vol. 204, p. 316.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 21 808 161).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yan, L., Si, L., Tao, Q. et al. The Continuous Synthesis of 2-(2'-Hydroxy-5'-Methylphenyl)Benzotriazole over Cu/γ-Al2O3. Kinet Catal 60, 635–641 (2019). https://doi.org/10.1134/S0023158419050124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158419050124