Abstract
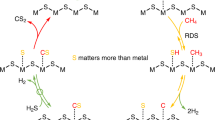
The thermodynamics of three pathways of the hydrogen sulfide decomposition reaction is considered. In the thermal process, the gas-phase dissociation of hydrogen sulfide yields hydrogen and diatomic singlet sulfur. Over sulfide catalysts, the reaction proceeds via the formation of disulfane (H2S2) as the key surface intermediate. This intermediate then decomposes to release hydrogen into the gas phase, and adsorbed singlet sulfur recombines into cyclooctasulfur. Over metal catalysts, H2S decomposes via dissociation into surface atoms followed by the formation of gaseous hydrogen and gaseous triplet disulfur. The last two pathways are thermodynamically forbidden in the gas phase and can take place at room temperature only on the surface of a catalyst. An alternative mechanism is suggested for hydrogen sulfide assimilation in the chemosynthesis process involving sulfur bacteria. To shift the hydrogen sulfide decomposition equilibrium toward the target product (hydrogen), it is suggested that the reaction should be conducted at room temperature as a three-phase process over a solid catalyst under a layer of a solvent that can dissolve hydrogen sulfide and sulfur. In this case, it is possible to attain an H2S conversion close to 100%. Therefore, hydrogen sulfide can be considered as an inexhaustible source of hydrogen, a valuable chemical and an environmentally friendly energetic product.
Similar content being viewed by others
References
Veziroglu, T.N. and Sakhin, Sh., Al’tern. Energ. Ekol., 2014, no. 2, p. 12.
Gol’tsov, V.A., Al’tern. Energ. Ekol., 2012, no. 4, p. 15.
James, O.O., Maity, S., Mesubi, M.A., Ogunniran, K.O., Siyanbola, T.O., Sahu, S., and Chaubey, R., Green Chem., 2011, vol. 13, p. 2272.
Zaman, J. and Chakma, A., Fuel Process. Technol., 1995, vol. 41, p. 159.
Luinstra, E.A., Hydrogen from H2S: Technologies and Economics, Calgary: Sulfotech Res., 1995.
Armor, J.N., Appl. Catal., A, 1999, vol. 176, p. 159.
Startsev, A.N., Zakharov, I.I., Voroshina, O.V., and Parmon, V.N., Dokl. Chem., 2004, vol. 399, part 1, p. 283.
Zakharov, I.I., Startsev, A.N., Voroshina, O.V., Pashigreva, A.V., Chashkova, N.A., and Parmon, V.N., Russ. J. Phys. Chem., 2006, vol. 80, no. 9, p. 1403.
Startsev, A.N., Kruglyakova, O.V., Chesalov, Yu.A., Ruzankin, S.Ph., Kravtsov, E.A., Larina, T.V., and Paukshtis, E.A., Top. Catal., 2013, vol. 56, p. 969.
Startsev, A.N. and Kruglyakova, O.V., J. Chem. Chem. Eng., 2013, vol. 7, p. 1007.
Startsev, A.N., Kruglyakova, O.V., Ruzankin, S.F., Bulgakov, N.N., Chesalov, Yu.A., Kravtsov, E.A., Zheivot, V.I., Larina, T.V., and Paukshtis, E.A., Zh. Fiz. Khim., 2014, vol. 88, no. 6, p. 943.
Swope, W.C., Lee, Y.-P., and Schaefer, H.F., J. Chem. Phys., 1979, vol. 70, no. 2, p. 947.
Barrow, R.F. and Parcq, R.P., in Elemental Sulfur: Chemistry and Physics, Meyer, B., Ed., New York: Interscience, 1965, p. 251.
Evans, W.H. and Wagman, D.D., J. Res. Natl. Bur. Stand., 1952, vol. 49, no. 3, p. 141.
Rau, H., Kutty, T.R.N., and de Carvalho, J.R.F.G., J. Chem. Thermodyn., 1973, vol. 5, p. 833.
Benson, S.W., Chem. Rev., 1978, vol. 78, no. 1, p. 23.
Abu-Yousef, I.A., J. Sulfur Chem., 2006, vol. 27, no. 1, p. 87.
Zysman-Colman, E. and Harpp, D.N., Heteroat. Chem., 2007, vol. 18, no. 5, p. 449.
Startsev, A.N., Bulgakov, N.N., Ruzankin, S.Ph., Kruglyakova, O.V., and Paukshtis, E.A., J. Sulfur Chem., 2015, vol. 36, no. 3, p. 234.
Startsev, A.N., Sul’fidnye katalizatory gidroochistki: Sintez, struktura, svoistva (Sulfide Hydrotreating Catalysts), Novosibirsk: GEO, 2007.
Startsev, A.N., Catal. Today, 2009, vol. 144, nos. 3–4, p. 350.
Zakharov, I.I. and Startsev, A.N., J. Phys. Chem. B, 2000, vol. 104, p. 9025.
Aleshina, G.I., Aksenov, D.G., and Startsev, A.N., Proc. Int. Symp. on Molecular Aspects of Catalysis by Sulfides, Novosibirsk, 1998, p. 100.
Startsev, A.N., Aleshina, G.I., and Aksenov, D.G., Proc. 2nd Int. Symp. on Molecular Aspects of Catalysis by Sulfides, Porqueroles, France, 2001, p. 33
Koestner, R.J., Salmeron, M., Kollin, E.B., and Gland, J.L., Surf. Sci., 1986, vol. 172, no. 3, p. 668.
Alfonso, D.R., Surf. Sci., 2008, vol. 602, p. 2758.
Rodriguez, J.A., Hrbek, J., Jirsak, M.T., Chaturvedi, S., and Maiti, A., J. Chem. Phys., 2000, vol. 113, no. 24, p. 11284.
Poelsema, B., Lenz, K., and Comsa, G., J. Phys.: Condens. Matter, 2010, vol. 22, p. 304006.
Startsev, A.N., Kruglyakova, O.V., Chesalov, Yu.A., Kravtsov, E.A., Serkova, A.N., Suprun, E.A., Salanov, A.N., and Zaikovskii, V.I., Russ. J. Phys. Chem. A, 2015, vol. 89, no. 1, p. 19.
Meyer, B., Sulfur, Energy, and Environment, Amsterdam: Elsevier, 1977.
Autotrophic Bacteria, Schlegel, H.G. and Bowien, B., Eds., Madison, Wis.: Science and Technology, 1989.
Kleinjan, W.E., de Keizer, A., and Janssen, A.J.H., Top. Curr. Chem., 2003, vol. 230, p. 167.
Robertson, L.A. and Kuenen, J.G., Prokaryotes, 2006, vol. 2, p. 985.
Sorokin, D.Yu., Banciu, H., Robertson, L.A., and Kuenen, J.G., Prokaryotes, 2006, vol. 2, p. 969.
Guerrero, R., Mas, J., and Pedros-Alio, C., Arch. Microbiol., 1984, vol. 137, p. 350.
Steudel, R., Holdt, G., Visscher, P.T., and van Gemerden, H., Arch. Microbiol., 1990, vol. 153, p. 432.
Janssen, A., de Keizer, A., van Aelst, A., Fokkink, R., Yangling, H., and Lettinga, G., Colloids Surf., B, 1996, vol. 6, p. 115.
Janssen, A.J.H., Lettinga, G., and de Keizer, A., Colloids Surf., A, 1999, vol. 151, p. 389.
Pickering, J., George, G.N., Yu, E.Y., Brune, D.C., Tuschak, Ch., Overmann, J., Beatty, J.T., and Prince, R.C., Biochemistry, 2001, vol. 40, p. 8138.
Pasteris, J.D., Freeman, J.J., Goffredi, S.K., and Buck, K.R., Chem. Geol., 2001, vol. 180, p. 3.
RF Patent 2261838, 2005.
Ukrainian Patent 81088, 2007.
Kazakh Patent 20390, 2008.
US Patent 7611685 B2, 2009.
Khairulin, S.R., Kuznetsov, V.V., Batuev, R.A., Teryaeva, T.N., Tryasunov, B.G., Garifullin, R.G., Filimonov, S.N., Sal’nikov, A.V., and Ismagilov, Z.R., Al’tern. Energ. Ekol., 2014, no. 3, p. 60.
Startsev, A.N., Kruglyakova, O.V., Chesalov, Yu.A., Paukshtis, E.A., Avdeev, V.I., Ruzankin, S.Ph., Zhdanov, A.A., Molina, I.Yu., and Plyasova, L.M., J. Sulfur Chem., 2016, vol. 37, no. 2, p. 229.
Steliou, K., Acc. Chem. Res., 1991, vol. 24, p. 341.
Harpp, D.N., Phosphorus, Sulfur Silicon Relat. Elem., 1997, vols. 120–121, p. 41.
Sangalov, Yu.A., Karchevskii, S.G., and Telyashev, R.G., Elementnaya sera (Elemental Sulfur), Ufa: GUP INKhP RB, 2014.
Boreskov, G.K., Geterogennyi kataliz (Heterogeneous Catalysis), Moscow: Nauka, 1986.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.N. Startsev, 2016, published in Kinetika i Kataliz, 2016, Vol. 57, No. 4, pp. 516–528.
Rights and permissions
About this article
Cite this article
Startsev, A.N. Low-temperature catalytic decomposition of hydrogen sulfide into hydrogen and diatomic gaseous sulfur. Kinet Catal 57, 511–522 (2016). https://doi.org/10.1134/S002315841604011X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002315841604011X