Abstract—
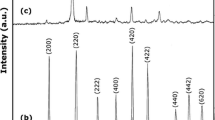
This paper examines processes for the preparation of metallic iridium nanoparticles under hydrothermal conditions. The reduction of aqueous potassium hexachloroiridate(IV) solutions with sodium tetrahydridoborate in acidic and alkaline media at temperatures from 130 to 180°C has been shown to take 2–30 min and result in the formation of fine metallic iridium powder with a characteristic mosaic structure. The average size of the iridium(0) nanoparticles varies from 8 to 300 nm, depending on synthesis conditions, and the crystallite size is no greater than 10 nm. According to low-temperature nitrogen gas adsorption measurements, the specific surface area of the materials prepared in acid solutions ranges from 1 to 10 m2/g, and that of the materials prepared in alkaline solutions reaches 25 m2/g. X-ray photoelectron spectroscopy results demonstrate that the surface of the 8-nm-diameter iridium nanoparticles is covered with an oxide film. As shown by differential scanning calorimetry and thermogravimetry in an argon atmosphere, the fraction of iridium oxide compounds in the material with a specific surface area of 25 m2/g does not exceed 5 wt %.




Similar content being viewed by others
REFERENCES
Chen, A. and Ostrom, C., Palladium-based nanomaterials: synthesis and electrochemical applications, Chem. Rev., 2015, vol. 115, pp. 11999–12044.https://doi.org/10.1021/acs.chemrev.5b00324
Axet, M.R. and Philippot, K., Catalysis with colloidal ruthenium nanoparticles, Chem. Rev., 2020, vol. 120, pp. 1085–1145.https://doi.org/10.1021/acs.chemrev.9b00434
Xu, H., Shang, H., Wang, C., and Du, Y., Low-dimensional metallic nanomaterials for advanced electrocatalysis, Adv. Funct. Mater., 2020, vol. 30, no. 50, paper 2006317.https://doi.org/10.1002/adfm.202006317
Maduraiveeran, G., Sasidharan, M., and Ganesan, V., Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications, Biosens. Bioelectron., 2018, vols. 103, pp. 113–129.https://doi.org/10.1016/j.bios.2017.12.031
He, L., Weniger, F., Neumann, H., and Beller, M., Synthesis, characterization, and application of metal nanoparticles supported on nitrogen-doped carbon: catalysis beyond electrochemistry, Angew. Chem., Int. Ed., 2016, vol. 55, no. 41, pp. 12582–12594.https://doi.org/10.1002/anie.201603198
Alekseev, E.S., Alentiev, A.Y., Belova, A.S., et al., Supercritical fluids in chemistry, Russ. Chem. Rev., 2020, vol. 89, pp. 1337–1427.https://doi.org/10.1070/RCR4932
Jovanovič, P., Hodnik, N., Ruiz-Zepeda, F., Arčon, I., Jozinovič, B., Zorko, M., Bele, M., Šala, M., Šelih, V.S., Hočevar, C., and Gaberšček, M., Electrochemical dissolution of iridium and iridium oxide particles in acidic media: transmission electron microscopy, electrochemical flow cell coupled to inductively coupled plasma mass spectrometry, and X-ray absorption spectroscopy study, J. Am. Chem. Soc., 2017, vol. 139, no. 36, pp. 12837–12846.https://doi.org/10.1021/jacs.7b08071
Lv, F., Feng, J., Wang, K., Dou, Z., Zhang, W., Zhou, J., Yang, C., Luo, M., Yang, Y., Li, Y., Gao, P., and Guo, S., Iridium–tungsten alloy nanodendrites as pH-universal water-splitting electrocatalysts, ACS Central Sci., 2018, vol. 4, no. 9, pp. 1244–1252.https://doi.org/10.1021/acscentsci.8b00426
Over, H., Fundamental studies of planar single-crystalline oxide model electrodes (RuO2, IrO2) for acidic water splitting, ACS Catal., 2021, vol. 11, no. 14, pp. 8848–8871.https://doi.org/10.1021/acscatal.1c01973
Wang, F., Kusada, K., Wu, D., Yamamoto, T., Toriyama, T., Matsumura, S., and Kitagawa, H., Solid-solution alloy nanoparticles of the immiscible iridium-copper system with a wide composition range for enhanced electrocatalytic applications, Angew. Chem., Int. Ed., 2018, vol. 57, pp. 4505–4509.https://doi.org/10.1002/anie.201800650
Liu, D., Chen, X., Xu, G., Guan, J., Cao, Q., Dong, B., and Mu, X., Iridium nanoparticles supported on hierarchical porous n-doped carbon: an efficient water-tolerant catalyst for bio-alcohol condensation in water, Sci. Rep., 2016, vol. 6, paper 21365.https://doi.org/10.1038/srep21365
Hara, M., Badam, R., Wang, G.J., Huang, H-H., and Yoshimura, M., Synthesis and evaluation of iridium oxide nanoparticle catalysts supported on nitrogen-doped reduced graphene oxides, ECS Trans., 2018, vol. 85, no. 11, pp. 27–35.https://doi.org/10.1149/08511.0027ecst
Rueping, M., Koenigs, R.M., Borrmann, R., Zoller, J., Weirich, T.E., and Mayer, J., Size-selective, stabilizer-free, hydrogenolytic synthesis of iridium nanoparticles supported on carbon nanotubes, Chem. Mater., 2011, vol. 23, no. 8, pp. 2008–2010.https://doi.org/10.1021/cm1032578
Belousov, O.V., Tarabanko, V.E., Borisov, R.V., Simakova, I.L., Zhyzhaev, A.M., Tarabanko, N., Isakova, V., Parfenov, V., and Ponomarenko, I.V., Synthesis and catalytic hydrogenation activity of Pd and bimetallic Au–Pd nanoparticles supported on high-porosity carbon materials, React. Kinet. Mech. Catal., 2019, vol. 127, no. 1, pp. 25–39.https://doi.org/10.1007/s11144-018-1430-0
Rodrigues, T.S., da Silva, A.G., and Camargo, P.H., Nanocatalysis by noble metal nanoparticles: controlled synthesis for the optimization and understanding of activities, J. Mater. Chem. A, 2019, vol. 7, pp. 5857–5874.https://doi.org/10.1039/C9TA00074G
Zahmakiran, M., Iridium nanoparticles stabilized by metal organic frameworks (IrNPs@ZIF-8): synthesis, structural properties and catalytic performance, Dalton Trans., 2012, vol. 41, pp. 12690–12696.https://doi.org/10.1039/C2DT31779F
Cui, M., Zhou, J., Zhao, Y., and Song, Q., Facile synthesis of iridium nanoparticles with superior peroxidase-like activity for colorimetric determination of H2O2 and xanthine, Sens. Actuators, B, 2017, vol. 43, pp. 203–210.https://doi.org/10.1016/j.snb.2016.11.145
Brown, A.L., Winter, H., Goforth, A.M., Sahay, G., and Sun, C., Facile synthesis of ligand-free iridium nanoparticles and their in vitro biocompatibility, Nanoscale Res. Lett., 2018, vol. 13, paper 208.https://doi.org/10.1186/s11671-018-2621-3
Kundu, S. and Liang, H., Shape-selective formation and characterization of catalytically active iridium nanoparticles, J. Colloid Interface Sci., 2011, vol. 354, pp. 597–606.https://doi.org/10.1016/j.jcis.2010.11.032
Hu, H., Xin, J.H., Wang, X., Miaoa, D., and Liua, Y., Synthesis and stabilization of metal nanocatalysts for reduction reactions – a review, J. Mater. Chem. A, 2015, vol. 3, no. 21, pp. 11157–11182.https://doi.org/10.1039/C5TA00753D
Goel, A. and Rani, N., Effect of PVP, PVA and POLE surfactants on the size of iridium nanoparticles, Open J. Inorg. Chem., 2012, vol. 2, no. 3, pp. 67–73.https://doi.org/10.4236/ojic.2012.23010
Bonet, F., Delmas, V., Grugeon, S., Urbina, R.H., Silvert, P.-Y., and Tekaia-Elhsissen, K., Synthesis of monodisperse Au, Pt, Pd, Ru and Ir nanoparticles in ethylene glycol, Nanostruct. Mater., 1999, vol. 11, pp. 1277–1284.https://doi.org/10.1016/S0965-9773(99)00419-5
Zhang, R., Liu, X., Shi, L., Jin, X., Dong, Y., Li, K., Zhao, X., Li, Q., and Deng, Y., A simple and fast method to synthesize cubic iridium nanoparticles with clean surface free from surfactants, Nanomaterials, 2019, vol. 9, no. 1, paper 76.https://doi.org/10.3390/nano9010076
Reshetnikova, E.A., Lisnevskaya, I.V., and Terekhin, A.I., Hydrothermal synthesis of sodium bismuth titanate ferroelectrics, Inorg. Mater., 2020, vol. 56, no. 1, pp. 83–90.https://doi.org/10.1134/S0020168520010136
Fesik, E.V., Buslaeva, T.M., Mel’nikova, T.I., Tarasova, L.S., and Laptenkova, A.V., Thermolysis of [Pd(NH3)4]Cl2–(NH4)2Cr2O7 mixtures in an inert atmosphere, Russ. J. Phys. Chem. A, 2019, vol. 93, no. 6, pp. 1011–1016.https://doi.org/10.1134/S0036024419060098
Zimbovskii, D.S., Churagulov, B.R., and Baranov, A.N., Hydrothermal synthesis of Cu2O films on the surface of metallic copper in a NaOH solution, Inorg. Mater., 2019, vol. 55, no. 6, pp. 582–585.https://doi.org/10.1134/S0020168519060177
Borisov, R.V., Belousov, O.V., Zhizhaev, A.M., Belousova, N.V., and Kirik, S.D., Carbon-supported palladium–gold bimetallic disperse systems formed in aqueous solutions at 110°C, Russ. J. Inorg. Chem., 2018, vol. 63, no. 3, pp. 308–313.https://doi.org/10.1134/S0036023618030038
Asanova, T.I., Asanov, I.P., Maksimovsky, E.A., Vasilchenko, D.B., and Korenev, S.V., Studying the process of (NH4)2[IrCl6] thermal decomposition by X-ray photoelectron spectroscopy and electron microscopy, J. Struct. Chem., 2020, vol. 61, no. 3, pp. 388–399.https://doi.org/10.1134/S0022476620030063
Plyusnin, P.E., Baidina, I.A., Shubin, Yu.V., and Korenev, S.V., Synthesis, crystal structure, and thermal properties of [Pd(NH3)4][AuCl4]2, Russ. J. Inorg. Chem., 2007, vol. 52, no. 3, pp. 371–377.https://doi.org/10.1134/S0036023607030138
Borisov, R.V., Belousov, O.V., Dorokhova, L.I., and Zhizhaev, A.M., Features of fine iridium powders dissolution in acidic media, J. Sib. Fed. Univ. Chem., 2017, vol. 10, no. 3, pp. 325–332.https://doi.org/10.17516/1998-2836-0029
Borisov, R.V., Belousov, O.V., Zhizhaev, A.M., Likhatskii, M.N., and Belousova, N.V., Synthesis of bimetallic nanoparticles Pd-Au and Pt-Au on carbon nanotubes in an autoclave, Russ. Chem. Bull., 2021, vol. 70, no. 8, pp. 1474–1482.https://doi.org/10.1007/s11172-021-3242-z
Goldberg, R.N. and Hepler, L.G., Thermochemistry and oxidation potentials of the platinum group metals and their compounds, Chem. Rev., 1968, vol. 68, no. 2, pp. 229–252.
Zakharov, Y.A., Pugachev, V.M., Bogomyakov, A.S., Ovcharenko, V.I., Korchuganova, K.A., Russakov, D.M., and Kolmykov, R.P., Influence of NicoreAushell nanoparticles' morphology on their magnetic properties, J. Phys. Chem. C, 2019, vol. 124, no. 1, pp. 1008–1019.https://doi.org/10.1021/acs.jpcc.9b07897
Kim, H.W., Shim, S.H., Myung, J.H., and Lee, C., Annealing effects on the structural properties of IrO2 thin films, Vacuum, 2008, vol. 82, no. 12, pp. 1400–1403.https://doi.org/10.1016/j.vacuum.2008.03.004
Buyanova, N.E. and Karnaukhov, A.P., Adsorbtsiya i poristost’ (Adsorption and Porosity), Dubinin, M.M., Ed., Moscow: Nauka, 1976.
Borisov, R.V., Belousov, O.V., and Irtyugo, L.A., Thermostimulated transformations of highly disperse powders of platinum group metals in an argon atmosphere, Russ. J. Phys. Chem. A, 2014, vol. 88, no. 10, pp. 1732–1738.https://doi.org/10.1134/S0036024414100069
Fonseca, G.S., Machado, G., Teixeira, S.R., Fecher, G.H., Morais, J., Alves, M.C., and Dupon, J., Synthesis and characterization of catalytic iridium nanoparticles in imidazolium ionic liquids, J. Colloid Interface Sci., 2006, vol. 301, no. 1, pp. 193–204.https://doi.org/10.1016/j.jcis.2006.04.073
Freakley, S.J., Ruiz-Esquius, J., and Morgan, D.J., The X-ray photoelectron spectra of Ir, IrO2 and IrCl3 revisited, Surf. Interface Anal., 2017, vol. 49, no. 8, pp. 794–799.https://doi.org/10.1002/sia.6225
Claudel, F., Dubau, L., Berthomé, G., Sola-Hernandez, L., Beauger, C., Piccolo, L., and Maillard, F., Degradation mechanisms of oxygen evolution reaction electrocatalysts: a combined identical-location transmission electron microscopy and X-ray photoelectron spectroscopy study, ACS Catal., 2019, vol. 9, no. 5, pp. 4688–4698.https://doi.org/10.1021/acscatal.9b00280
Lozanov, V.V., Il’in, I.Yu., Morozova, N.B., Trubin, S.V., and Baklanova, N.I., Chemical vapor deposition of an iridium phase on hafnium carbide and tantalum carbide, Russ. J. Inorg. Chem., 2020, vol. 65, no. 11, pp. 1781–1788.https://doi.org/10.1134/S0036023620110108
ACKNOWLEDGMENTS
In this study, we used equipment at the Krasnoyarsk Regional Shared Research Facilities Center, Krasnoyarsk Scientific Center (Federal Research Center), Siberian Branch, Russian Academy of Sciences.
Funding
This work was supported by the Russian Federation Ministry of Science and Higher Education as part of the state research target for the Institute of Chemistry and Chemical Technology, Siberian Branch, Russian Academy of Sciences, project no. 0287-2021-0014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Borisov, R.V., Belousov, O.V., Zhizhaev, A.M. et al. Characterization of Metallic Iridium Nanoparticles Synthesized under Hydrothermal Conditions. Inorg Mater 58, 215–222 (2022). https://doi.org/10.1134/S0020168522020030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168522020030