Abstract
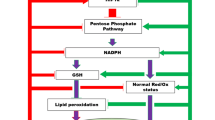
Potential mechanisms for the realization of the neuroprotective action of nicotinamide during acute lethal short-term hypoxia were evaluated based on current knowledge about its effect on metabolic processes. Attention was drawn to the role of mitochondrial dysfunction and excitotoxicity followed by axonal degeneration and apoptosis of neurocytes and neuroglia during the development of an inflammatory response in trauma and cerebral ischemia. A decrease in the level of ATP in the cells during hypoxia affects the generation of the mitochondrial membrane potential, promotes an increase in membrane permeability, the release of NAD from mitochondria, the entry of sodium into the cell, and the development of intracellular edema. Activation of poly (ADP-ribose)-polymerase 1, induced by DNA damage during reoxygenation, reduces the level of NAD in the cell as a substrate for its reaction and causes dysfunction of the respiratory mitochondrial complex. High doses of nicotinamide have neuroprotective properties in traumatic brain injury, ischemia and stroke, as well as in neurodegenerative Alzheimer’s, Parkinson’s and Huntington’s diseases. It is generally accepted that the neuroprotective effect of nicotinamide, firstly, is because, being a substrate for the synthesis of nicotinamide mononucleotide and NAD+, it can maintain and prevent the NAD+ content decrease under conditions of acute hypoxia. Secondly, nicotinamide, being a blocker of poly (ADP-ribose) polymerase 1, can provide the required level of NAD+ in the cell and reduce its use in the reactions with this polymerase. Thirdly, nicotinamide is a substrate for NAD+ synthesis and maintains the NAD+/NADH complex, which is important for the functioning of the antioxidant system of the mitochondrial respiratory chain under conditions of acute hypoxia. It is doubtful that these mechanisms are sufficient to implement the action of nicotinamide for the reason that there was no decrease in the level of NAD in mitochondria during the death of animals under conditions of short-term lethal hypoxia. A variant of the mechanism of nicotinamide action through GABA/benzodiazepine receptors, which causes inhibition of the activation of neurocyte glutamate receptors during acute hypoxia, was considered. In hypoxia, there is an excessive hyperactivation of glutamate receptors and the development of acute cellular hypoxia, which leads to cell death from overexcitation (excitotoxicity). Nicotinamide reduces the death of neurocytes from excitotoxicity by acting on benzodiazepine receptors. GABA agonists prevent the effect of glutamate during excitotoxicity in this way.
Similar content being viewed by others
REFERENCES
R. Moretti and C. Peinkhofer, Int. J. Mol. Sci. 20, 5797 (2019). https://doi.org/10.3390/ijms20225797
R. A. Fricker, E. L. Green, S. I. Jenkins, and S. M. Griffin, Int. J. Tryptophan Res. 11, 1178646918776658 (2018). https://doi.org/10.1177/1178646918776658
E. S. Hwang and S. B. Song, Biomolecules 10, 687 (2020).https://doi.org/10.3390/biom10050687
J. R. Tribble, A. Otmani, S. Sun, et al., Redox Biol. 43, 101988 (2021). https://doi.org/10.1016/j.redox.2021.101988
T. A. Voronina, Rev. Clin. Pharmacol. Drug Ther. 14, 63 (2016). https://doi.org/10.17816/RCF14163-70
M. E. Watts, R. Pocock, and C. Claudianos, Front. Mol. Neurosci. 11, 216 (2018). https://doi.org/10.3389/fnmol.2018.00216
S. Y. Ng and A. Y. W. Lee, Front. Cell. Neurosci. 13, 528 (2019). https://doi.org/10.3389/fncel.2019.00528
G. Qi, Y. Mi, and F. Yin, Front. Physiol. 10, 1531 (2020). https://doi.org/10.3389/fphys.2019.01531
M. S. Sekhon, P. N. Ainslie, and D. E. Griesdale, Crit. Care 21, 90 (2017). https://doi.org/10.1186/s13054-017-1670-9
K. Shetty, F. Galeffi, and D. A. Turner, Neurobiol. Dis. 62, 469 (2014). https://doi.org/10.1016/j.nbd.2013.10.025
C. Chinopoulos, Exp. Neurol. 327, 113218 (2020). https://doi.org/10.1016/j.expneurol.2020.113218
M. V. Vasin, I. B. Ushakov, and I. V. Bukhtiyarov, Biol. Bull. 45, 73 (2018). https://doi.org/10.1134/S1062359017060115
P. Belenguer, J. M. N. Duarte, P. F. Schuck, and G. C. Ferreira, Neurotoxic. Res. 36, 219 (2019). https://doi.org/10.1007/s12640-019-00061-7
E. Marutani, M. Morita, S. Hirai, et al., Nat. Commun. 12, 3108 (2021). https://doi.org/10.1038/s41467-021-23363-x
N. Klimova, A. Fearnow, and T. Kristian, Brain Sci. 10, 449 (2020). https://doi.org/10.3390/brainsci10070449
O. P. Mishra, W. Akhter, Q. M. Ashraf, and M. Delivoria-Papadopoulos, Neuroscience 119, 1023 (2003). https://doi.org/10.1016/s0306-4522(03)00166-0
P. Jagtap and C. Szabo, Nat. Rev. Drug Discovery 4, 421 (2005). https://doi.org/10.1038/nrd1718
R. P. Strosznajder, K. Czubowicz, H. Jesko, and J. B. Strosznajder, Mol. Neurobiol. 41, 187 (2010). https://doi.org/10.1007/s12035-010-8124-6
K. Erdélyi, P. Pacher, L. Virag, and C. Szabo, Int. J. Mol. Med. 32, 339 (2013). https://doi.org/10.3892/ijmm.2013.1397
S. Tanuma, A. Sato, T. Oyama, et al., Curr. Protein Pept. Sci. 17, 668 (2016). https://doi.org/10.2174/1389203717666160419150014
W. Ying, Antioxid. Redox Signal. 10, 179 (2008). https://doi.org/10.1089/ars.2007.1672
T. Neira-Pena, E. Rojas-Mancilla, V. Munoz-Vio, et al., Neurotoxic. Res. 27, 453 (2015). https://doi.org/10.1007/s12640-015-9517-0
B. M. Emerling, F. Weinberg, and C. Snyder. Free Radic. Biol. Med. 46, 1386 (2009).
J. M. Marti, A. Garcia-Diaz, and D. Delgado-Bellido, Redox Biol. 41, 101885 (2021). https://doi.org/10.1016/j.redox.2021.101885
E. Dengler, Int. J. Mol. Sci. 21 (7), 2428 (2020). https://doi.org/10.3390/ijms21072428
R. C. Rabinovitch, B. Samborska, B. Faubert, et al. Cell Rep. 21, 1 (2017). https://doi.org/10.1016/j.celrep.2017.09.026
C. Cantó, Z. Gerhart-Hines, J. N. Feige, et al., Nature 458 (7241), 1056 (2009). https://doi.org/10.1038/nature07813
J. Brandauer, S. G. Vienberg, M. A. Andersen, et al. J. Physiol. 591, 5207 (2013). https://doi.org/10.1113/jphysiol.2013.259515
A. Y. Sun and D. S. Cheng, Zhongguo Yaoli Xuebao 19, 104 (1998).
M. V. Vasin, T. V. Ryasina, and Yu. N. Chernov, Tsitologiya 41, 812 (1999).
T. V. Ryasina, M. V. Vasin, L. D. Smirnov, et al., Usp. Gerontol., No. 6, 67 (2001).
J. Yang, L. K. Klaidman, A. Nalbandian, et al., Neurosci. Lett. 333, 91 (2002). https://doi.org/10.1016/s0304-3940(02)01005-4
J. Yang, L. K. Klaidman, M. L. Chang, et al., Pharmacol., Biochem. Behav. 73, 901 (2002). https://doi.org/10.1016/s0091-3057(02)00939-5
M. L. Chang, J. Yang, S. Kem, et al., Neurosci. Lett. 322, 137 (2002). https://doi.org/10.1016/s0304-3940(01)02520-4
L. Klaidman, M. Morales, S. Kem, et al., J. Pharmacol. 69, 150 (2003). https://doi.org/10.1159/000072668
M. R. Hoane, S. L. Akstulewicz, and J. J. Toppen, Neurotrauma 20, 1189 (2003). https://doi.org/10.1089/089771503770802871
M. R. Hoane, J. L. Pierce, M. A. Holland, and G. D. Anderson., Neuroscience 154, 861 (2008). https://doi.org/10.1016/j.neuroscience.2008.04.044
M. R. Hoane, J. L. Pierce, N. A. Kaufman, and J. E. Beare, Oxid. Med. Cell Longevity 1, 46 (2008). https://doi.org/10.4161/oxim.1.1.6694
A. M. Goffus, G. D. Anderson, and M. R. Hoane, Oxid. Med. Cellul. Longevity 3, 145 (2010). https://doi.org/10.4161/oxim.3.2.11315
C. Vonder Haar, G. D. Anderson, and M. R. Hoane, Behav. Brain Res. 224, 311 (2011). https://doi.org/10.1016/j.bbr.2011.06.009
J. H. Park, A. Long, K. Owens, and T. Kristian, Neurobiol. Dis. 95, 102 (2016). https://doi.org/10.1016/j.nbd.2016.07.018
C. C. Wei, Y. Y. Kong, G. Q. Li, et al., Sci. Rep. 7, 717 (2017). https://doi.org/10.1038/s41598-017-00851-z
N. Klimova, A. Fearnow, A. Long, and T. Kristian, Exp. Neurol. 325, 113144 (2020). https://doi.org/10.1016/j.expneurol.2019.113144
J. Zhang, Y. Hong, W. Cao, et al., Front. Mol. Neurosci. 12, 108 (2019). https://doi.org/10.3389/nmol.2019.00108
Y. F. Lai, L. Wang, and W. Y Liu, Eur. Rev. Med. Pharmacol. Sci. 23, 1797 (2019). https://doi.org/10.26355/eurrev_201902_17143
K. Maiese, Curr. Neurovasc. Res. 17, 765 (2020). https://doi.org/10.2174/1567202617999201111195232
N. Klimova, A. Long, and T. Kristian, J. Neurosci. Res. 97, 975 (2019). https://doi.org/10.1002/jnr.24397
Yu. N. Chernov, M. V. Vasin, and I. B. Ushakov, Eksp. Klin. Farmakol. 84, 32 (2021). https://doi.org/10.30906/0869-2092-2021-84-3-8-10
D. Belov Kirdajova, J. Kriska, J. Tureckova, and M. Anderova, Front. Cell Neurosci. 14, 51, (2020). https://doi.org/10.3389/fncel.2020.00051
D. W. Choi, Front. Neurosci. 14, 579953 (2020). https://doi.org/10.3389/fnins.2020.579953
C. Chen, X. Zhou, J. He, et al., Oxid. Med. Cell Longevity 2019, 4028394 (2019). https://doi.org/10.1155/2019/4028394
C. Vinnakota, K. Govindpani, W. P. Tate, et al., Int. J. Mol. Sci. 21, 3284 (2020). https://doi.org/10.3390/ijms21093284
H. Song, S. M. Mylvaganam, J. Wang, et al., Front. Cell Neurosci. 12, 278, (2018). https://doi.org/10.3389/fncel.2018.00278
H. Mohler, P. Polc, R. Cumin, et al., Nature 278, 563 (1979).
J. Prousky, J. Orthomol. Med. 19, 104 (2004).
M. Slomka, E. Zieminska, E. Salinska, and W. Lazarewicz, Folia Neuropathol. 46, 69 (2008).
D. Mayor and M. Tymianski, Neuropharmacology 134 (Pt B), 178 (2018)https://doi.org/10.1016/j.neuropharm.2017.11.050
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals or human subjects performed by any of the authors.
Additional information
Translated by E. Puchkov
Abbreviations: ROS, reactive oxygen species; PARP-1, poly (ADP-ribose) polymerase 1.
Rights and permissions
About this article
Cite this article
Vasin, M.V., Ilyin, L.A. & Ushakov, I.B. Analysis of the Effect of Exogenous Nicotinamide on Bioenergetic Processes in the Brain During Acute Hypoxia. BIOPHYSICS 67, 637–641 (2022). https://doi.org/10.1134/S0006350922040224
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350922040224