Abstract
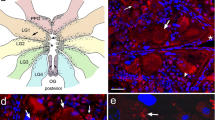
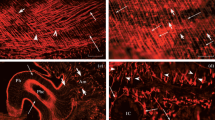
The present work focuses on the study of localization of peptidergic neurons and muscle fibers of body wall musculature in planarians Girardia tigrina and Polycelis tenuis with the use of immunohistochemistry, immunocytochemistry, fluorescent microscopy, and confocal laser scanning microscopy. A close spatial relationship between FMRFamide-immunopositive nerve fibers and myofilaments is shown. Such localization of peripheral peptidergic nerve fibers may suggest an important role FMRF-like neuropeptides play in the regulation of muscle function. Physiological analysis of the muscle cells isolated from the planarian Procerodes littoralis confirmed that native flatworm FMRF-like peptides GYIRF and YIRF have the inducing effect on muscle contractions in planarians. It was found that dihydropyridine calcium channel blockers (nicardipine, nitrendipine, and nifedipine), as well as ryanodine, an antagonist of the endoplasmic reticulum calcium channels, inhibited peptide-induced muscle contraction. The blockers of the intracellular calcium ions reuptake (thapsigargin and cyclopiazonic acid) decreased the number of peptide-induced muscular responses. The findings suggest that FMRF-like peptide-induced muscle contraction is dependent on calcium ions from both extracellular pool and intracellular stores. The results demonstrate the presence of different receptors and ion channels controlling muscle contractions in flatworms.






Similar content being viewed by others
REFERENCES
C. Shaw, A. G. Maule, and D. W. Halton, Int. J. Parasitol. 26 (4), 335 (1996).
P. McVeigh, G. R. Mair, L. Atkinson, et al., Int. J. Parasitol. 39, 1243 (2009).
R. N. Johnston, C. Shaw, D. W. Halton, et al., Biochem. Biophys. Res. Commun. 209, 689 (1995).
R. N. Johnston, C. Shaw, D. W. Halton, et al., J. Neurochem. 67, 814 (1996).
G. Maule, D. W. Halton, and L. Thim, Biochem. Biophys. Res. Commun. 193, 1054 (1993).
D. A. Price and M. J. Greenberg, Science 197 (4304), 670 (1977).
N. D. Kreshchenko, Biochemistry (Moscow). Suppl. Ser. A: Membr. Cell Biol. 8 (1), 89 (2014).
O. O. Tolstenkov, L. Akimova, N. B. Terenina, and M. K. S. Gustafsson, Parasitol. Res. 111, 1977 (2012).
M. Almuedo-Castillo, X. Crespo, F. Seebeck, et al., PLoS Genet. 10 (6), e1004400 (2014).
L.-Ch. Cheng, K. C. Tu, C. W. Seidel, et al., Dev. Biol. 433, 357 (2018).
J. Baguna, Semin. Cell Dev. Biol. 87, 3 (2019).
V. V. Novikov, I. M. Sheiman, and E. E. Fesenko, Bioelectromagnetics 29 (5), 387 (2008). https://doi.org/10.1002/bem.20407
P. G. Barghouth, M. Thiruvalluvan, and N. J. Oviedo, Biochim. Biophys. Acta, Biomembr. 1848, 2629 (2015). https://doi.org/10.1016/j.bbamem.2015.02.024
F. Durant, J. Bischof, Ch. Fields, et al., Biophys. J. 116, 948 (2019).
T. Nogi, D. Zhang, J. D. Chan, et al., PLoS Negl. Trop. Dis. 3 (6), e464 (2009).
J. J. Collins and Ph. A. Newmark, PLoS Pathog. 9 (7), e1003396 (2013).
N. J. Wheeler, P. N. Agbedanu, M. J. Kimber, et al., Parasitol. Vectors 8, 34 (2015).
E. I. Maciel, C. Jiang, P. G. Barghouth, et al., Dev. Comp. Immunol. 93, 18 (2019).
F. Cebria, Front. Cell Dev. Biol. 4, 8 (2016). https://doi.org/10.3389/fcell.2016.00008
N. D. Kreshchenko, Biophysics (Moscow) 62 (2), 271 (2017).
D. W. Halton and A. G. Maule, Can. J. Zool. 82, 316 (2004).
R. Pascolini, F. Panara, I. Di Rosa, et al., Cell. Tissue Res., 499 (1992).
R. Pascolini, F. Panara, G. Gabbiani, et al., Boll. Zool. 60 (4), 403 (1993). https://doi.org/10.1080/11250009309355848
M. H. Wahlberg, Cell. Tissue Res. 291 (3), 561 (1998).
G. R. Mair, D. W. Halton, A. G. Maule, and C. Shaw, Parasitol. Today 14, 73 (1998).
K. L. Blair, T. A. Day, M. C. Lewis, et al., Parasitology 102, 251 (1991).
C. G. Moneypenny, N. Kreshchenko, T. A. Day, et al., Parasitology 122, 447 (2001).
N. Kreshchenko, T. A. Day, D. W. Halton, and A. G. Maule, Acta Parasitol. 45 (3), 256 (2000).
N. D. Kreshchenko, M. Totten, A. G. Maule, et al., in Proc. Int. Symp. “Biological Motility: New Trends in Research,” Abstracts of Papers (Pushchino, 2001), pp. 82–83.
N. B. Terenina, N. D. Kreshchenko, N. V. Mochalova, and S. O. Movsesyan, Helminthologia 55 (3), 185 (2018).
O. O. Tolstenkov, V. V. Prokofiev, N. B. Terenina, and M. K. S. Gustafsson, Parasitol. Res. 108, 1219 (2011). https://doi.org/10.1007/s00436-010-2166-6
M. Reuter, M. K. S. Gustafsson, J. Lang, and C. J. P. Grimmelkuijzen, Zoomorphology 109, 303 (1990).
G. R. Mair, R. N. Johnston, D.W. Halton, et al., Zoomorphology 116 (4), 213 (1996).
K. Mäntylä, D. W. Halton, M. Reuter, et al., Hydrobiologia 383, 167 (1998).
M. Reuter, M. K. S. Gustafsson, I. M. Sheiman, et al., Invertebrate Neurosci. 1, 133 (1995).
N. Kreshchenko, M. Reuter, I. Sheiman, et al., Invertebr. Reprod. Dev. 35 (2), 109 (1999).
M. Reuter, M. K. S. Gustafsson, K. Mäntylä, and C. J. P. Grimmelikhuijzen, Zoomorphology 116, 111 (1996).
F. Cebria, Neurosci. Res. 61, 375 (2008).
K. Mäntylä, M. Reuter, D. W. Halton, et al., Acta Zool. (Stockholm) 79 (1), 1 (1998b).
C. G. Moneypenny, A. G. Maule, C. Shaw, et al., Parasitology 115, 281 (1997).
G. Hrĉkova, S. Velebny, D. W. Halton, et al., Int. J. Parasitol. 34, 83 (2004).
N. J. Marks, S. Johnson, D. W. Halton, et al., Parasitology 113, 394 (1996).
M. K. Graham, I. Fairweather, and J. G. McGeown, Parasitology 114, 455 (1997).
K. L. Blair and P. A. V. Anderson, Parasitology 109, 325 (1994).
T. A. Day, A. G. Maule, C. Shaw, et al., Parasitology 109, 455 (1994).
T. A. Day, A. G. Maule, C. Shaw, and R. A. Pax, Peptides 18, 917 (1997).
T. A. Day, J. Haithcock, M. Kimber, and A. G. Maule, Parasitology 120, 417 (2000).
P. Cobbett and T. A. Day, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol. 134, 593 (2003).
ACKNOWLEDGMENTS
I am grateful to Prof. D.W. Halton and Prof. A.G. Maule from Queen’s University of Belfast, Northern Ireland, United Kingdom for the opportunity to work in the laboratory and their supervision and to Dr. A. Mousley for help in mastering muscle cell culture techniques.
The study was performed using the equipment of the Sector of Optical Microscopy and Spectrophotometry of the Core Facilities of the Pushchino Scientific Center for Biological Research (Pushchino, Russia).
Funding
The study of muscle contraction physiology was supported by the Royal Society Fellowship Program, Great Britain. Immunocytochemical studies for the identification of FMRF-like peptides and the histochemical study of musculature in planarians were supported by the Russian Foundation for Basic Research (project no. 18-04-00349a).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The author declares that no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Translated by M. Batrukova
Abbreviations: PBS buffer—4% paraformaldehyde in 0.1 M phosphate buffer; PBST—PBS buffer supplemented with 0.3% Triton X-100; FMRF-ip—FMRF-immunopositive nerve fibers.
Rights and permissions
About this article
Cite this article
Kreshchenko, N.D. A Study of the Mechanisms of Action of FMRF-Like Peptides in Inducing Muscle Contraction in Planarians (Platyhelminthes). BIOPHYSICS 66, 472–482 (2021). https://doi.org/10.1134/S000635092103009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000635092103009X