Abstract
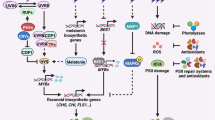
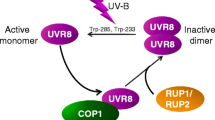
UVB radiation (290-320 nm) causes diverse effects in plant cells that vary with the fluence rate of exposure. High fluence rates of UVB radiation cause damage to DNA and formation of reactive oxygen species in mitochondria and chloroplasts, which lead to oxidation of membrane proteins and lipids and inhibition of cellular functions. In response to oxidative stress, mitochondrial transmembrane potential dissipates, resulting in cytochrome c release and activation of metacaspases. This leads to the apoptosis-like cell death. The signaling mechanism based on UVB DNA damage includes check-point activation, cell-cycle arrest, and finally programmed cell death with characteristic DNA fragmentation and morphological hallmarks typical of apoptotic cells. Recently, it was shown that among the components of this signaling mechanism the transcriptional factor SOG1 (suppressor of gamma response 1) plays a key role in regulation of programmed cell death in plants. In contrast to its damaging effects, UVB radiation at low fluence rates can act as a regulatory signal that is specifically perceived by plants to promote acclimation and survival in sunlight. The protective action of UVB is based on expression of various genes, including those encoding flavonoid synthesis enzymes that provide a UVB-absorbing sunscreen in epidermal tissues and DNA photorepair enzymes. These processes are mediated by the UVB photoreceptor UVR8, which has been recently characterized at the molecular level. Now progress is made in uncovering the UVR8-mediated signaling path-way mechanism in the context of UVB photon perception and revealing the biochemical components of the early stages of light signal transduction. In this review, attention is focused on the achievements in studying these UVB-induced signaling processes.
Similar content being viewed by others
Abbreviations
- COP1:
-
E3 ubiquitin ligase
- CPD:
-
cyclobutane pyrimidine dimers
- HY5:
-
transcription factor
- PCD:
-
programmed cell death
- ROS:
-
reactive oxygen species
- RUP1/RUP2:
-
repressors of photomorphogenesis 1 and 2
- SOG1:
-
suppressor of gamma response 1 (transcription factor)
- SPA1:
-
suppressor of phyA-1
- UVB/UVA:
-
ultraviolet B/ultraviolet A
- UVR8:
-
UV resistance locus 8 (UVB receptor protein)
References
Paul, N. D., and Gwynn, J. D. (2003) Ecological roles of solar UV radiation: towards an integrated approach, Trends Ecol. Evol., 18, 48–55.
Caldwell, M. M., Bornman, J. F., Ballare, C. L., Flint, S. D., and Kulandaivelu, G. (2007) Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors, Photochem. Photobiol. Sci., 6, 252–266.
McKenzie, R. L., Aucamp, P. J., Bais, A. F., Bjorn, L. O., and Iljas, M. (2007) Changes in biologically–active ultravi–olet radiation reaching the Earth’s surface, Photochem. Photobiol. Sci., 6, 218–231.
Frohnmeyer, H., and Staiger, D. (2003) Ultraviolet–B radi–ation–mediated responses in plants. Balancing damage and protection, Plant Physiol., 133, 1420–1428.
Ulm, R., and Nagy, F. (2005) Signaling and gene regulation in response to ultraviolet light, Curr. Opin. Plant Biol., 8, 477–482.
Fraikin, G. Y., Strakhovskaya, M. G., and Rubin, A. B. (2013) Biological photoreceptors of light–dependent regu–latory processes, Biochemistry (Moscow), 78, 1238–1253.
Rizzini, L., Favory, J. J., Cloix, C., Faggionato, D., Hara, A. O., Kaiserli, E., Baumeister, R., Schafer, E., Nagy, F., and Jenkins, G. I. (2011) Perception of UV–B by the Arabidopsis UVR8 protein, Science, 332, 103–106.
Jenkins, G. I. (2009) Signal transduction in responses to UV–B radiation, Annu. Rev. Plant Biol., 60, 407–431.
Balare, C. L., Caldwell, M. M., Flint, S. D., Robinson, S. A., and Bornman, J. F. (2011) Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change, Photochem. Photobiol. Sci., 10, 226–241.
Morales, L. O., Brosche, M., Vainonen, J., Jenkins, G. I., Wargent, J. J., Sipari, N., Strid, A., Lindfors, A. V., Tegelberg, R., and Aphalo, P. J. (2013) Multiple roles for UV resistance locus8 in regulating gene expression and metabolite accumulation in Arabidopsis under solar ultravi–olet radiation, Plant Physiol., 161, 744–759.
Brosche, M., and Strid, A. (2003) Molecular events follow–ing perception of ultraviolet–B radiation by plants, Physiol. Plant., 117, 1–10.
Cadet, J., Douki, T., and Ravanat, J.–L. (2015) Oxidatively generated damage to cellular DNA by UVB and UVA radi–ation, Photochem. Photobiol., 91, 140–155.
Ciccia, A., and Elledge, S. J. (2010) The DNA damage response: making it safe to play with knives, Mol. Cell, 40, 179–204.
Mannuss, A., Trapp, O., and Puchta, H. (2012) Gene reg–ulation in response to DNA damage, Biochim. Biophys. Acta, 1819, 154–165.
Reape, T. J., and McCabe, P. F. (2008) Apoptotic–like pro–grammed cell death in plants, New Phytol., 180, 13–26.
Lytvyn, D. I., Yemets, A. I., and Blume, Y. B. (2010) UV–B overexposure induces programmed cell death in a BY–2 tobacco cell line, Environ. Exp. Bot., 68, 51–57.
Gao, C., Xing, D., Li, L., and Zhang, L. (2008) Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet–C overexposure, Planta, 227, 755–767.
Lam, E., and Zhang, Y. (2012) Regulating the reapers: acti–vating metacaspases for programmed cell death, Trends Plant Sci., 17, 487–494.
Brown, B. A., Cloix, C., Jiang, G. H., Kaiserli, E., Herzyk, P., Kliebenstein, D. I., and Jenkins, G. I. (2005) UV–B spe–cific signaling component orchestrates plant UV protec–tion, Proc. Natl. Acad. Sci. USA, 102, 18225–18230.
Brown, B. A., Headland, L. R., and Jenkins, G. I. (2009) UV–B action spectrum for UVR8–mediated HY5 transcript accumulation in Arabidopsis, Photochem. Photobiol., 85, 1147–1155.
Favory, J. J., Stec, A., Gruber, H., Rizzini, L., Oravecz, A., Funk, M., Albert, A., Cloix, C., Jenkins, G. I., and Oakeley, E. A. (2009) Interaction of COP1–UVR8 regulates UVB–induced photomorphogenesis and stress acclimation in Arabidopsis, EMBO J., 28, 591–601.
Heijde, M., and Ulm, R. (2012) UV–B photoreceptor–mediated signaling in plants, Trends Plant Sci., 17, 230–237.
Christie, J. M., Arvai, A. S., Baxter, K. J., Heilmann, M., Pratt, A. J., O’Hara, A., Kelly, S. M., Hothorn, M., Smith, B. O., Hitomi, K., Jenkins, G. I., and Getzoff, E. D. (2012) Plant UVR8 photoreceptor senses UV–B by tryptophan–mediated disruption of cross–dimer salt bridges, Science, 335, 1492–1496.
Wu, D., Hu, Q., Yan, Z., Chen, W., Yan, C., Huang, X., Zhang, J., Yang, P., and Wang, J. (2012) Structural basis of ultraviolet–B perception by UVR8, Nature, 484, 214–219.
O’Hara, A., and Jenkins, G. I. (2012) In vivo function of tryptophans in the Arabidopsis UV–B photoreceptor UVR8, Plant Cell, 24, 3755–3766.
Brown, B. A., and Jenkins, G. I. (2008) UV–B signaling pathways with different fluence–rate response profiles are distinguished in mature Arabidopsis leaf tissue by require–ment for UVR8, HY5, and HYH, Plant Physiol., 146, 576–588.
Stracke, R., Favory, J. J., Gruber, H., Bartelniewoehner, L., Bartels, S., Binkert, M., Funk, M., Weisshaar, B., and Ulm, R. (2010) The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet–B radiation, Plant Cell Environ., 33, 88–103.
Cloix, C., Kaiserli, E., Heilmann, M., Baxter, K. J., Brown, B. A., O’Hara, A., Smith, B. O., Christie, J. M., and Jenkins, G. I. (2012) C–terminal region of the UV–B photoreceptor UVR8 initiates signaling through interaction with the COP1 protein, Proc. Natl. Acad. Sci. USA, 109, 16366–16370.
Buer, C. S., Imin, N., and Djordjevic, M. A. (2010) Flavonoids: new roles for old molecules, J. Integrat. Plant Biol., 52, 98–111.
Gruber, H., Heijde, M., Heller, W., Albert, A., Seidlitz, H. K., and Ulm, R. (2010) Negative feedback regulation of UV–B–induced photomorphogenesis and stress acclimation in Arabidopsis, Proc. Natl. Acad. Sci. USA, 107, 20132–20137.
Heijde, M., and Ulm, R. (2013) Reversion of the Arabidopsis UV–B photoreceptor UVR8 to the homodimer–ic ground state, Proc. Natl. Acad. Sci. USA, 110, 1113–1118.
Heilmann, M., and Jenkins, G. I. (2013) Rapid reversion from monomer to dimer regenerates the ultraviolet–B pho–toreceptor UV resistance locus 8 in intact Arabidopsis plants, Plant Physiol., 161, 547–555.
Jiang, L., Wang, Y., Li, Q. F., Bjorn, L. O., He, J. X., and Li, S. S. (2012) Arabidopsis STO/BBX24 negatively regu–lates UV–B signaling by interacting with COP1 and repress–ing HY5 transcriptional activity, Cell Res., 22, 1046–1057.
Lian, H.–L., He, S.–B., Zhang, Y.–C., Zhu, D.–M., Zhang, J.–Y., Jia, K.–P., Sun, S.–X., Li, L., and Yang, H.–Q. (2011) Blue–light–dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism, Genes Dev., 25, 1023–1028.
Sheerin, D. J., Zur Oven–Krockhaus, S., Enderle, B., Zhu, L., Johnen, P., Schleifenbaum, F., Stierhof, Y. D., Hug, E., and Hiltbrunner, A. (2015) Light–activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP/SPA complex, Plant Cell, 27, 189–201.
Gardner, G., Lin, C., Tobin, E. M., Loehrer, H., and Brinkman, D. (2009) Photobiological properties of the inhibition of etiolated Arabidopsis seedling growth by ultra–violet–B irradiation, Plant Cell Environ., 32, 1573–1583.
Ulm, R. (2003) Molecular genetics of genotoxic stress sig–naling in plants, Topics Curr. Genet., 4, 217–240.
Herrlich, P., Karin, M., and Weiss, C. (2008) Supreme EnLIGHTement: damage recognition and signaling in the mammalian response, Mol. Cell, 29, 279–290.
Gonzales Besteiro, M. A., Bartels, S., Albert, A., and Ulm, R. (2011) Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV–B stress tolerance, independent of the UVR8 photoreceptor pathway, Plant J., 68, 727–737.
Bartels, S., Anderson, J. C., Gonzales Besteiro, M. A., Carreri, A., Hirt, H., Buchala, A., Metraux, J. P., Peck, S. C., and Ulm, R. (2009) MAP kinase phosphatase1 and pro–tein tyrosine phosphatase1 are repressors of salicylic acid synthesis and SNC1–mediated responses in Arabidopsis, Plant Cell, 21, 2884–2897.
Zhou, C., Cai, Z., Guo, Y., and Gan, S. (2009) An Arabidopsis mitogen–activated protein kinase cascade MPK9–MPK6 plays a role in leaf senescence, Plant Physiol., 150, 167–177.
Fomicheva, A. S., Tuzhikov, A. I., Beloshistov, R. E., Trusova, S. V., Galiullina, R. A., Mochalova, L. V., Chichkova, N. V., and Vartapetian, A. B. (2012) Programmed cell death in plants, Biochemistry (Moscow), 77, 1452–1464.
Yoshiyama, K. O., Sakaguchi, K., and Kimura, S. (2013) DNA damage response in plants: conserved and variable response compared to animals, Biology, 2, 1338–1356.
Nawkar, G. M., Maibam, P., Park, J. H., Sahi, V. P., Lee, S. Y., and Kang, C. H. (2013) UV–induced cell death in plants, Int. J. Mol. Sci., 14, 1608–1628.
Ries, G., Heller, W., Puchta, H., Sandermann, H., Seidlitz, H. K., and Hohn, B. (2000) Elevated UV–B radiation reduces genome stability in plants, Nature, 406, 98–101.
Sinha, R. P., and Hader, D. P. (2002) UV–induced DNA damage and repair: a review, Photochem. Photobiol. Sci., 1, 225–236.
Zhou, B. B., and Elledge, S. J. (2000) The DNA damage response: putting checkpoints in perspective, Nature, 408, 433–439.
Sancar, A., Lindsey–Boltz, L. A., Unzal–Kacmaz, K., and Linn, S. (2004) Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints, Annu. Rev. Biochem., 73, 39–85.
Teranishi, M., Nakamura, K., Morioka, H., Yamamoto, K., and Hidema, J. (2008) The native cyclobutane pyrimi–dine dimer photolyase of rice is phosphorylated, Plant Physiol., 146, 1941–1951.
Liu, Z., Hossain, G. S., Islas–Osuna, M. A., Mitchell, D. L., and Mount, D. W. (2000) Repair of UV damage in plants by nucleotide excision repair: Arabidopsis UVH1 DNA repair gene is a homolog of Saccharomyces cerevisiae Rad1, Plant J., 21, 519–528.
Dubest, S., Gallego, M. E., and White, C. I. (2002) Role of the AtRad1 endonuclease in homologous recombination in plants, EMBO Rep., 3, 1049–1054.
Sancar, A. (2008) Structure and function of photolyase and in vivo enzymology: 50th anniversary, J. Biol. Chem., 283, 32153–32157.
Nakajima, S., Sugiyama, M., Iwai, S., Hitomi, K., Otoshi, E., Kim, S., Jiang, C. Z., Todo, T., Britt, A. B., and Yamamoto, K. (1998) Cloning and characterization of a gene (UVR3) required for photorepair of 6–4 photoproducts in Arabidopsis thaliana, Nucleic Acids Res., 26, 638–644.
Cimprich, K. A., and Cortez, D. (2008) ATR: an essential regulator of genome integrity, Nat. Rev. Mol. Cell. Biol., 9, 616–627.
Shiloh, Y., and Ziv, Y. (2013) The ATM protein kinase: reg–ulating the cellular response to genotoxic stress, and more, Nat. Rev. Mol. Cell. Biol., 14, 197–210.
Helton, E. S., and Chen, X. (2007) P53 modulation of the DNA damage response, J. Cell. Biochem., 100, 883–896.
Culligan, K., Tissier, A., and Britt, A. (2004) ATR regulates a G2–phase cell–cycle checkpoint in Arabidopsis thaliana, Plant Cell, 16, 1091–1104.
Sakamoto, A. N., Lan, V. T., Puripunyavanich, V., Hase, Y., Yokota, Y., Shikazono, N., Nakagawa, M., Narumi, I., and Tanaka, A. (2009) UVB–hypersensitive mutant in Arabidopsis thaliana is defective in the DNA damage response, Plant J., 60, 509–517.
Jiang, L., Wang, Y., Bjorn, L. O., and Li, S. (2011) UV–B induced DNA damage mediates expression changes of cell cycle regulatory genes in Arabidopsis root tips, Planta, 233, 831–841.
Takahashi, S., Kojo, K. H., Kutsuna, N., Endo, M., Toki, S., Isoda, H., and Hasezawa, S. (2015) Differential responses to high–and low–dose ultraviolet–B stress in tobacco Bright Yellow–2 cells, Front. Plant Sci., 6, 1–10.
Furukawa, T., Curtis, M. J., Tominey, C. M., Duong, Y. H., Wilcox, B. W. L., Aggoune, D., Hays, J. B., and Britt, A. B. (2010) A shared DNA–damage response pathway for induc–tion of stem–cell death by UVB and by gamma irradiation, DNA Repair, 9, 940–948.
Curtis, M. J., and Hays, J. B. (2011) Cooperative respons–es of DNA–damage–activated protein kinases ATR and ATM and DNA translesion polymerases to replication–blocking DNA damage in a stem–cell niche, DNA Repair, 10, 1272–1281.
Yoshiyama, K., Conclin, P. A., Huefner, N. D., and Britt, A. B. (2009) Suppressor of gamma response 1 (sog1) encodes a putative transcription factor governing multiple responses to DNA damage, Proc. Natl. Acad. Sci. USA, 106, 12843–12848.
Yoshiyama, K., Kobayashi, J., Ogita, N., Ueda, M., Kimura, S., Maki, H., and Umeda, M. (2013) ATM–medi–ated phosphorylation of SOG1 is essential for the DNA damage response in Arabidopsis, EMBO Rep., 14, 817–822.
Biever, J. J., Brinkman, D., and Gardner, G. (2014) UV–B inhibition of hypocotyls growth in etiolated Arabidopsis thaliana seedlings is a consequence of cell cycle arrest initiat–ed by photodimer accumulation, J. Exp. Bot., 65, 2949–2961.
Mackerness, S., John, C. F., Jordan, B., and Thomas, B. (2001) Early signaling components in ultraviolet–B responses: distinct roles for different reactive oxygen species and nitric oxide, FEBS Lett., 489, 237–242.
Kalbina, I., and Strid, A. (2006) The role of NADPH oxi–dase and MAP kinase phosphatase in UV–B–dependent gene expression in Arabidopsis, Plant Cell Environ., 29, 1783–1793.
Yao, N., Eisfelder, B. J., Marvin, J., and Greenberg, J. T. (2004) The mitochondrion–an organelle commonly involved in programmed cell death in Arabidopsis thaliana, Plant J., 40, 596–610.
Danon, A., Rotari, V. I., Gordon, A., Mailhac, N., and Gallois, P. (2004) Ultraviolet–C overexposure induces pro–grammed cell death in Arabidopsis, which is mediated by caspase–like activities and which can be suppressed by cas–pase inhibitors, p35 and defender against apoptotic death, J. Biol. Chem., 279, 779–787.
Zhang, L., Xu, Q., Xing, D., Gao, C., and Xiong, H. (2009) Real time detection of caspase–3–like protease acti–vation in vivo using fluorescence resonance energy transfer during plant programmed cell death induced by ultraviolet C overexposure, Plant Physiol., 150, 1773–1783.
Watanabe, N., and Lam, E. (2005) Two Arabidopsis meta–caspases AtMCP1 band AtMCP2b are arginine/lysine–spe–cific cysteine proteases and activate apoptosis–like cell death in yeast, J. Biol. Chem., 280, 14691–14699.
He, R., Drury, G. E., Rotari, V. I., Gordon, A., Willer, M., Farzaneh, T., Woltering, E. J., and Gallois, P. (2008) Metacaspase–8 modulates programmed cell death induced by ultraviolet light and H2O2 in Arabidopsis, J. Biol. Chem., 283, 774–783.
Jiang, L., Wang, Y., Bjorn, L. O., and Li, S. (2009) Arabidopsis radical–induced cell death is involved in UV–B signaling, Photochem. Photobiol. Sci., 8, 838–846.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G. Ya. Fraikin, 2018, published in Biokhimiya, 2018, Vol. 83, No. 7, pp. 972–980.
Rights and permissions
About this article
Cite this article
Fraikin, G.Y. Signaling Mechanisms Regulating Diverse Plant Cell Responses to UVB Radiation. Biochemistry Moscow 83, 787–794 (2018). https://doi.org/10.1134/S0006297918070027
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297918070027