Abstract
The processes of the biocatalytic acylation of 1-methyl-5-mercapto-1,2,3,4-tetrazolil-7-amino-cephalosporanic acid (7-TMCA) and 7-aminocephalosporanic acid (7-ACA) by methyl ester of mandelic acid (MEMA) were optimized with an immobilized cephalosporin-acid synthetase as the biocatalyst. Under optimized conditions in water-organic medium containing 43% (vol/vol) of ethylene glycol at 30°С with a spontaneous pH gradient in the range of 8.0–6.0, the following yields of biocatalytic transformations were reached: (80.8 ± 1.9)% for 7‑TMCA acylation (at a concentration of 100–120 mМ) resulted in cefamandole (CFM) production, and (88.6 ± 2.0)% for 7-ACA acylation of (at concentration of 140–170 mМ) resulted in a semiproduct of CFM (S-p CFM) formation. In the second process, the concentration of the target β-lactam product in the final reaction mixture is one and a half times higher than that with the first one. In light of the undoubted environmental benefits of the chemical transformation of S-p CFM to CFM over the process of the chemical production of 7-TMCA from 7-ACA, we conclude that the second pathway of combined chemical and biocatalytic CFM synthesis is preferable.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
INTRODUCTION
Cefamandol (CFM) is a second-generation, parenteral, cephalosporin antibiotic with a broad spectrum of action and high resistance to the action of plasmid β-lactamases, including staphylococcal penicillinases [1]. The advantages of CFM include its high efficiency against infections caused by Haemophilus influenzae, as well as the ability to combine this cephalosporin with penicillins and aminoglycosides.
The biocatalytic synthesis of semisynthetic β-lactam antibiotics is a promising direction in the field of organic synthesis, an alternative to chemical synthesis in drug production. The biocatalytic synthesis of β‑lactams is the subject of numerous studies [2–5], since it makes it possible to avoid the use of toxic reagents, large volumes of organic solvents, low reaction temperatures, and the purification of intermediate products in the multistage process of chemical synthesis of β-lactam antibiotics. Thus, the use of biocatalytic transformation to obtain β-lactam antibiotics can certainly reduce the burden on the environment and reduce production costs [6, 7].
For biocatalytic processes of the transformation of organic compounds, the used enzymes are not in dissolved form but in the form of heterogeneous biocatalysts (BCs), immobilized (bound to solid carriers) enzymes. Due to the high specificity of the enzyme, it is possible to obtain target products of high purity [8–10].
The biocatalytic synthesis of the antibiotic cefazolin (CEZ) via the acylation of 7-aminocephalosporanic acid (7-ACA), as well as the synthesis of the CEZ intermediate (S-p CEZ) via the acylation of 3-[(5-methyl-1,3,4-thiadiazol-2-yl)-thiomethyl]-7-aminocephalosporanic acid (TDA), was studied and optimized earlier [11] via comparison of the efficiency of two chemical-biocatalytic pathways for synthesis. Based on the results, it was concluded that it is preferable to use the biocatalytic acylation of 7-ACA to develop a chemical-biocatalytic technology for CEZ production.
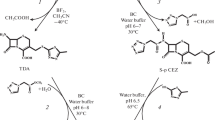
This work compares two biocatalytic pathways for the synthesis of another β-lactam antibiotic, CFM. Cefamandole can be synthesized by the two chemical-biocatalytic routes shown in Fig. 1. The traditional chemical-biocatalytic approach to CFM synthesis is based on the fact that, first, the necessary substituent is introduced chemically into the C3‑position of 7-ACA (Fig. 1, transformation 1), and the amino group of the resulting 1-methyl-5-mercapto-1,2,3,4-tetrazolyl-7-aminocephalosporanic acid derivative (7-TMCA) is then biocatalytically acylated to form CFM (Fig. 1, transformation 2).
An alternative chemical-biocatalytic approach is the use of biocatalysis for the acylation of the amino group at the C7-position of 7-ACA (Fig. 1, transformation 3), followed by the chemical transformation of the intermediate CFM (S-p CFM) into the target antibiotic (Fig. 1, transformation 4).
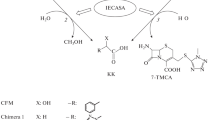
The biocatalytic synthesis of CFM and S-p CFM was carried out with the acyl transfer method (kinetically controlled synthesis) [6, 10, 11], with 7-TMCA and 7-ACA as the key amino acids (KAs) and mandelic acid methyl ester (MEMA) as the acylating agent (AA). Immobilized cephalosporin-acid synthetase (IECASA) was used as a biocatalyst. The enzyme catalyzes three competitive reactions: the synthesis of CFM or S-p CFM and two side hydrolytic reactions, namely, hydrolysis of the acylating agent MEMA and cleavage of the acylamide bond of the target product with the formation of a side product, mandelic acid (MA). The yield of the β-lactam antibiotic is kinetically controlled, since it depends on the rate of all occurring reactions.
The enzyme cephalosporin-acid synthetase (CASA EC 3.5.1.11) is highly specific for cephalosporin-acid synthesis [6, 12]. It was found [12] that CASA is encoded by a gene that is a homolog of the broadly specific penicillin acylase (PA) gene from Escherichia coli. In this work, the CASA enzyme produced by the recombinant E. coli strain VKPM V-12316 and immobilized via covalent binding to an epoxy-activated macroporous carrier was used as a BC [5].
The goal of this work is to optimize the key stages of two chemical-biocatalytic routes for CFM production, namely, the processes of biocatalytic acylation of 7-TMCA with the formation of CFM and 7-ACA with the formation of an intermediate S-p CFM catalyzed by IECASA. The yield of the target product of biocatalytic transformation with respect to KAs and the achieved concentration of the β-lactam product in the final reaction mixture were used as optimization criteria. Optimization was carried out according to such parameters as the initial KA concentration and the composition of the reaction medium, namely, the concentration of dihydric alcohol ethylene glycol (EG), which is miscible with water.
MATERIALS AND METHODS
The following materials were used in the work: commercial samples of 7-ACA from Anhui BBCA Pharmaceutical Co., LTD (China) with a 93% purity as determined with high-performance liquid chromatography (HPLC); 96.5% pure (determined with HPLC) 7-TMCA, Shandong Sihuan Pharmaceutical Co., Ltd., (China); 99% pure MEMA (Sigma-Aldrich, United States, determined with HPLC); 99% pure cefamandole sodium salt (Sigma-Aldrich, United States, determined with HPLC); 99% pure MA (Sigma-Aldrich, United States, determined with HPLC); EG (ReaKhimLab, Russia), GOST 19770-83, 99.9% concentrate. A laboratory sample of the S-p CFM standard (97% purity, determined with HPLC) was kindly provided by the Sichuan Industrial Institute of Antibiotics (China).
Preparation of the IECASA biocatalyst. The IECASA biocatalyst was obtained according to the procedure described earlier [5, 11] via immobilization of the CASA enzyme from the recombinant E. coli strain VKPM V-12316 on a Seplite LX-1000EP microporous, epoxy-activated support (Sunresin New Materials, China). An IECASA sample with a synthetase activity of 340 IU/g of wet BC with a solids content of 37% was obtained for work.
The synthetase activity of a heterogeneous IECASA biocatalyst sample was determined from the initial reaction rate of CEZ synthesis from TDA and 1(H)-tetrazolylacetic acid methyl ester (METzAA) with HPLC control of the CEZ content in the reaction mixture.
For 1 international unit (IU) of the synthetase activity of the sample in the CEZ synthesis reaction, we took the amount of the BC that catalyzes the formation of 1 μmol CEZ per 1 min in a solution containing 60 mM TDA and 240 mM METzAA at a temperature of 30°C and an initial pH of 7.5 [5, 11].
HPLC analysis. Analysis of the reaction mixtures obtained during the biocatalytic synthesis of CFM and S-p CFM, as well as mixtures obtained from the study of substrate solubility, was carried out by HPLC in isocratic mode on a Gilson chromatograph (United States) with a Spherisorb ODS chromatographic column, 250 × 4 mm with a particle size of 7.5 µm. A mixture consisting of 0.05 M ammonium phosphate buffer at a pH of 4.0 and methanol in various ratios depending on the studied process was used as the mobile phase (Table 1). The flow rate of the mobile phase is 1.0 mL/min. Detection of peaks of analytes was carried out spectrophotometrically at a wavelength of 218 or 254 nm. Conditions for HPLC analysis and the retention times of the analytes (RT) are presented in Table 1.
Synthesis of CFM and S-p CFM catalyzed by IECASA. The biocatalytic synthesis of CFM and S‑p CFM was carried out via acylation of the corresponding KA (7-TMCA or 7-ACA) with mandelic acid methyl ester. The synthesis process was carried out in a glass reactor with a capacity of 75 mL equipped with a paddle mechanical stirrer and systems to maintain the temperature and pH. To prepare a solution of substrates in a reactor at a temperature of 30 ± 1°C, a sample of KA was suspended in 0.3 M sodium phosphate buffer (PB), pH 8.3, with or without EG. The KA was dissolved under pH control with constant stirring and portionwise addition of 2 M NaOH until the complete dissolution of KA. In this case, the pH did not exceed 8.0. After the dissolution of KA, a portion of MEMA was added. The solution was stirred for 3–5 min until the complete or partial dissolution of AA, and the process of biocatalytic synthesis was started via the addition of the amount of IECASA necessary to create the required content of the active enzyme in the reaction mixture (CE = 10–30 IU/mL). The pH value decreased in the course of the reaction due to MA formation. The process of CFM or S-p CFM synthesis was carried out with stirring of the reaction mixture, temperature 30 ± 1°C in a spontaneously established pH gradient. After a pH value of 6.0 was reached, it was maintained via the addition of 2.0 M NaOH. The synthesis was stopped via the separation of IECASA with vacuum filtration on a porous glass filter.
To study the dynamics of the synthesis process, a sample of the reaction mixture was taken every 5–10 min and the content of four components was monitored via HPLC: KA (7-TMCA or 7-ACA), MEMA acylating agent, target β-lactam product (CFM or S-p CFM) and MA by-product. The synthesis was carried out until a stable plateau was reached on the curve of the dependence of the relative concentration of the target product on time (the plateau of the maximum concentration of the target product, \({\text{C}}_{{{\text{prod}}}}^{{\max }}\), mM). The relative content of the target product (CFM or S-p CFM) at the current time was calculated regarding the content of KA (7-TMCA or 7-ACA) in the reaction mixture at the initial time (\({\text{C}}_{{{\text{KA}}}}^{{\text{o}}},\) mM). The maximum degree of KA transformation into the target product (maximum yield of the target product, \({{{{\eta }}}^{{{\text{max}}}}},\) %) was calculated with the formula \({{{{\eta }}}^{{{\text{max}}}}} = \frac{{{\text{C}}_{{{\text{prod}}}}^{{{\text{max}}}}}}{{{\text{C}}_{{{\text{KA}}}}^{{\text{o}}}}} \times 100\) (average data on the plateau).
RESULTS AND DISCUSSION
Solubility of substrates for the biocatalytic synthesis of CFM and S-p CFM. The solubility of KA and AA is an important tool for the selection of the optimal conditions for the processes of the biocatalytic synthesis of β-lactams [14]. The solubility of 7-TMCA, 7-ACA, and MEMA was studied under conditions that simulate the parameters of biocatalytic processes: 30°C, 0.3 M PB, and an EG initial concentration (CEG, % vol/vol) of 0 or 43%. The experiments were carried out with the saturation method described earlier [5, 14, 16]. The results are presented in Table 2.
The pH-independent solubility of MEMA that is not an electrolyte, was studied at a pH of 6.5, which ensured the practical absence of spontaneous hydrolysis of this ester during the experiments. Table 2 shows that the presence of EG significantly affected the MEMA solubility. Thus, the solubility of MEMA in an aqueous buffer medium at CEG = 0% vol/vol was 130 mM, and it reached 360 mM at CEG = 43% vol/vol).
The effect of the pH on the solubility of 7-TMCA and 7-ACA amino acids at slightly acidic, neutral, and slightly alkaline pH was studied, and the ionization constants of the carboxyl (рK2) and amino groups (рK1), as well as the characteristic solubility (S±), were determined.
For the amino acids 7-TMCA and 7-ACA, the dependence of solubility (S, mM) on the pH is described by the equation
At neutral and alkaline pH values upon complete deprotonation of the carboxyl group of the amino acid, the following equation can be used:
At an acidic pH with complete protonation of the amino group of the amino acid, the applicable equation is
where [H+] is the concentration of hydrogen ions in the solution at a given pH, mM; K2 is the ionization constant of the carboxyl group of the amino acid, mM; K1 is the ionization constant of the amino group of an amino acid, mM; and S± is the solubility of an individual electrically neutral zwitterionic form of an amino acid (characteristic electrolyte solubility), mM.
Figures 2a and 3a show the linearization of experimental data in the coordinates of the equation (1.1, S vs. 1/[H+]), which was obtained from the study of the effect of pH on the solubility of amino acids 7-TMCA and 7-ACA at neutral and slightly alkaline pH values at CEG = 0% and CEG = 43% (vol/vol), respectively. The linearization in the coordinates of equation (1.2, S vs. [H+]) of the experimental data obtained in the study of the effect of pH on the solubility of amino acids at neutral and slightly acidic pH at CEG = 0% and CEG = 43% (vol/vol) is shown in Figs. 2b and 3b, respectively.
Dependences of 7-TMCA solubility on pH in 0.3 M PB at 30°C: (a) the coordinates of equation (1.1), (b) the coordinates of equation (1.2). 1, CEG = 0% (vol/vol); 2, CEG = 43% (vol/vol).
Dependences of 7-ACA solubility on pH in 0.3 M PB at 30°C: (a) in the coordinates of equation (1.1), (b) in the coordinates of equation (1.2). 1, CEG = 0% (vol/vol); 2, CEG = 43% (vol/vol).
The calculated values of the ionization constants and characteristic solubility for 7-TMCA and 7-ACA are given in Table 2. Ethylene glycol had practically no effect on the pK2 value of 7-TMCA and 7-ACA, but it increased the pK1 value, especially in the case of 7-ACA (by 0.5 pH units). The characteristic solubilities for both amino acids decreased in the presence of EG: by 1.5 times for 7-TMCA and by two times for 7-ACA.
According to equation (1) with the constants presented in Table 2, the theoretical curves of the dependence of the solubility of 7-TMCA and 7-ACA on pH were calculated at CEG = 0% and CEG = 43% (vol/vol) (curves 1–4 in Fig. 4). Figure 4 also shows the solubility of MEMA in an aqueous medium (curve 5) and in the presence of EG (curve 6).
Theoretical curves of the dependence of the substrate solubility on pH calculated with the constants presented in Table 2 (30°C, 0.3 M PB). Curves 1–4 were calculated with equation (1). 1, 7-TMCA at CEG = 0%; 2, 7-TMCA at CEG = 43%; 3, 7-ACA at CEG = 0%; 4 – 7-ACA at CEG = 43%; 5, MEMA at CEG = 0%; 6, MEMA at CEG = 43%.
The effect of EG on the solubility of KAs is different: the solubility of 7-TMCA in the presence of EG decreased (curves 1 and 2, Fig. 4), while the solubility of 7-ACA increased significantly (curves 3 and 4, Fig. 4). At the same time, in the entire studied pH range, the solubility of 7-ACA significantly exceeded the solubility of 7-TMCA, both in an aqueous medium (curves 3 and 1, Fig. 4) and in the presence of EG (curves 4 and 2, Fig. 4). In a operating pH range of 6.0–7.0, where CASA is highly active and stable [5], the solubility of 7-TMCA in an aqueous medium varied from 4.5 to 43 mM, while the solubility of 7-ACA exceeded it by more than ten times, ranging from 55 to 570 mM. (calculation according to equation (1)). The presence of EG in the medium (CEG = 43%, vol/vol) increased the gap in the KA solubility in a pH range of 6.0–7.0 by up to 80 times: the solubility of 7-TMCA changed from 3.0 to 30 mM, and the solubility of 7-ACA from 250 to 2500 mM (calculated according to equation (1)). In the presence of EG (CEG = 43%, vol/vol), the MEMA solubility increased by 2.8 times as compared to the aqueous medium (CEG = 0% (vol/vol)) (curves 6 and 5 in Fig. 4, Table 2). In the study of CFM synthesis, in order to achieve an initial 7-TMCA concentration in the reaction mixture that exceeds its solubility in a working range of pH 6.0–7.0, the substrate solution was prepared at a pH of about 8.0. In the course of 7‑TMCA acylation with mandelic acid methyl ester, the pH decreased due to the formation of MA; however, 7‑TMCA did not precipitate due to the supersaturation effect, as previously described for TDA during the synthesis of CEZ in [5].
Thus, considering the possibility of the creation of high initial concentrations of KA and AA in the reaction medium, the biocatalytic process of 7-ACA acylation with the formation of S-p CFM proceeding in a medium containing EG is preferred.
Optimization of biocatalytic synthesis of CFM and S-p CFM catalyzed by IECASA. Ethylene glycol can be used as a component of an aqueous organic medium to increase the yield of the target β-lactam in acyl transfer synthesis. The observed effect is a consequence of a decrease in water activity in the presence of organic solvents, which entails a decrease in the rate of unproductive hydrolytic processes. In this case, the hydrolysis of both the acylating agent and the target products slows [16, 17]. A number of studies have demonstrated a significant increase in the yield of amino-β-lactam antibiotics ampicillin [18–20] and cephalexin [21–23] during their kinetically controlled synthesis catalyzed by variously immobilized PA from E. coli due to the presence of EG in reaction medium. It was shown that the solvent had no inactivating effect on PA from E. coli [18, 19]. In this work, EG was used to increase the yield of CFM and S-p CFM, which belong to the class of β-lactam acids, in the processes of their biocatalytic synthesis catalyzed by BC based on the recombinant CASA enzyme, which is homologous to PA from E. coli [12].
The processes of the biocatalytic acylation of 7‑TMCA and 7-ACA with mandelic acid methyl ester were carried out under the following conditions: 30°C, 0.3 M PB, Хо = 3.3 M/M (molar excess of AA over KA, \({{{\text{X}}}^{{\text{o}}}} = \frac{{{\text{C}}_{{{\text{AA}}}}^{{\text{o}}}}}{{{\text{C}}_{{{\text{KA}}}}^{{\text{o}}}}}\) ), spontaneous pH gradient (spontaneous decrease in pH from the value at which the substrates are dissolved to pH 6.0, and maintenance of this pH value until the end of the process).
These conditions were chosen for the IECASA biocatalyst during the optimization of the synthesis of CEZ and S-p CEZ [5, 11]. In this work, two parameters were varied in the optimization of the synthesis of CFM and Sp CFM: the initial concentration of KA (\({\text{C}}_{{{\text{7}} - {\text{TMCA}}}}^{{\text{o}}}\) = 60–120 mM or \({\text{C}}_{{{\text{7}} - {\text{ACA}}}}^{{\text{o}}}\) = 60–170 mM) and the concentration of EG in the reaction mixture (СEG = 0–43%, vol/vol). The IECASA biocatalyst was added in an amount that ensured the content of the active enzyme in the reaction mixture CE = 10–30 IU/mL, so that the duration of the process, including the plateau of the maximum concentration of the product, was 70–90 min.
We note that EG inhibits not only side processes of hydrolysis that occur during acyl transfer synthesis, but also (to a lesser extent) the target process of synthesis [17]. It was shown that the initial rate of CFM accumulation in the course of its biocatalytic synthesis decreased by two times at СEG = 43% (vol/vol) as compared to the initial rate of the process in an aqueous medium. The processes of biocatalytic synthesis of CFM and S-p CFM at EG concentrations of more than 43% have not been studied, since their implementation for a time not exceeding 90 min requires an IECASA content in the reaction mixture of more than 100 mg/mL, which hinders mass transfer processes.
In each experiment on β-lactam synthesis, the composition of the reaction mixture was dynamically controlled for all components (KA, AA, target product, MA), and the balances for β-lactam and MA were calculated. The results of two experiments carried out at СEG = 43% are shown in Figures 5 and 6 for the biocatalytic acylation of 7-TMCA and 7-ACA, respectively, at the maximum KA concentration used for each process: \({\text{C}}_{{{\text{7}} - {\text{TMCA}}}}^{{\text{o}}}\) = 120 mM (Fig. 5) and \({\text{C}}_{{{\text{7}} - {\text{ACA}}}}^{{\text{o}}}\) = 170 mM (Fig. 6).
Changes in the composition of the reaction mixture (relative concentrations, %) with time during CFM synthesis catalyzed by IECASA (30°C, 0.3 M PB, CE = 30 IU/mL, \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\) = 120 mM, \({\text{C}}_{{{\text{AA}}}}^{{\text{o}}}\) = 400 mM, Xo = 3.3 M/M, CEG = 43%, vol/vol, spontaneous pH gradient in the pH range of 8.0–6.0). 1, 7-TMCA; 2, CFM calculated relative to \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\); 3, balance (%) for β-lactam, the sum of the relative concentrations of 7-TMCA and CFM; 4, MEMA; 5, MA, calculated relative to \({\text{C}}_{{{\text{AA}}}}^{{\text{o}}}\); 6, balance (%) by MA, the sum of the relative concentrations of CFM, MEMA, and MA.
Changes in the composition of the reaction mixture (relative concentrations, %) with time during the synthesis of S-p CFM catalyzed by IECASA (30°C, 0.3 M PB, CE = 30 IU/mL, \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\) = 170 mM, \({\text{C}}_{{{\text{AA}}}}^{{\text{o}}}\) = 560 mM, Xo = 3.3 M/M, CEG = 43%, vol/vol, spontaneous pH gradient in the pH range of 8.0–6.0): 1, 7-ACA; 2, S-p CFM calculated relative to \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}};\) 3, balance (%) for β-lactam, the sum of the relative concentrations of 7-ACA and S-p CFM; 4, MEMA; 5, MA, calculated in relation to \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\); 6, balance (%) by MA, the sum of the relative concentrations of S-p CFM, MEMA, and MA.
In both processes, a long plateau was observed on the curves of accumulation of the target product (Figs. 5 and 6, curves 2). The maximum yields of CFM and S-p CFM, which were calculated as the average value at the plateau, were 81.3 ± 2.2 and 88.8 ± 2.3%, respectively, in these experiments. A feature of the S-p CFM synthesis process, the results of which are shown in Fig. 6, was the use of AA at an initial concentration of 560 mM, which exceeds the solubility of MEMA. Due to the incomplete dissolution of MEMA during the first 15 min, there was a lack of balance not only for MA (Fig. 6, curve 6) but also for β-lactam (Fig. 6, curve 3). This can be explained by the fact that some of the 7-ACA also precipitated in the presence of undissolved AA crystals. Further, both balances reached 100%, i.e., both substrates were completely dissolved; in addition, there were no side processes in the system, including those involving the β-lactam core.
Based on the results of all experiments on the synthesis of CFM and S-p CFM catalyzed by IECASA, the dependences of the maximum yield of the reaction product (ƞmax, %) on EG concentration (CEG, % vol/vol) and the initial KA concentration (\({\text{C}}_{{{\text{KA}}}}^{{\text{o}}},\) mM) shown in Fig. 7 and 8, were respectively plotted.
Dependence of the maximum yield of the β-lactam product (ηmax, %) on the concentration of ethylene glycol (CEG, % (vol/vol)) in the reaction mixture in the acyl transfer synthesis catalyzed by IECASA (30°C, 0.3 M PB, Xo = 3.3 M/M, spontaneous pH gradient): 1, CFM synthesis from 7-TMCA and MEMA at CE = 10–20 IU/mL; \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\)= 60 mM; 2, synthesis of S-p CFM from 7-ACA and MEMA at CE = 30 IU/mL; \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\) = 150 mM.
Dependence of the maximum yield of the β-lactam product (ηmax, %) on the initial KA concentration (\({\text{C}}_{{{\text{KA}}}}^{{\text{o}}},\) mM) in the acyl transfer synthesis catalyzed by IECASA (30°C, 0.3 M PB, Xo =3.3 M/M, CE = 20–30 IU/mL, spontaneous pH gradient, CEG = 43%, vol/vol): 1, CFM synthesis from 7-TMCA and MEMA; 2, synthesis of S-p CFM from 7-ACA and MEMA.
The use of an aqueous organic medium containing EG made it possible to increase the CFM yield (Fig. 7, Curve 1) from 60% (CEG = 0% (vol/vol)) to 70% (CEG = 43%, vol/vol). With an increase in the EG concentration from 0 to 43%, the S-p CFM yield increased from 80 to 88% (Fig. 7, curve 2).
For further studies of the processes of biocatalytic synthesis of CFM and S-p CFM catalyzed by IECASA, an aqueous-organic medium containing EG at a concentration of 43% (vol/vol) was chosen, since it provided the highest yield of target products. The dependences of the maximum yield of each of the processes (ηmax, %) on the initial KA concentration (\({\text{C}}_{{{\text{KA}}}}^{{\text{o}}},\) mM) are shown in Fig. 8. The dependence of the CFM yield on \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\) was studied in the range of 50–120 mM (Fig. 8, curve 1), since the solubility of 7‑TMCA under experimental conditions did not make it possible to achieve a higher KA concentration than 120 mM in the initial reaction mixture. The dependence of the S-p CFM yield on the initial 7-ACA concentration was studied in the range \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\) = 40–170 mM (Fig. 8, curve 2). The solubility of 7-ACA under experimental conditions at CEG = 43% (vol/vol), pH 6.0–8.0 is very high (Fig. 4, curve 4); however, the range of used \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\) at Хо = 3.3 M/M is limited by the solubility of MEMA.
The dependences of the product yield on \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\) have the form of curves with saturation (Fig. 8). The CFM yield increased monotonically with an increase in the 7-TMCA concentration up to \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\) = 100 mM and reached the average value 80.8 ± 1.9% in the range of 100–120 mM. The highest yield of the product of the S-p CFM synthesis was achieved in the range \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\) from 140 to 170 mM, and its average value in this range was 88.6 ± 2.0%.
Based on the results, the following optimal conditions for the IECASA-catalyzed CFM and S-p CFM synthesis processes were chosen: 30°C, 0.3 M PB, СEG = 43% (vol/vol), CE = 30 IU/mL, spontaneous pH gradient in the pH range from 8.0 to 6.0, Xo = 3.3 M/M, \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\) = 100–120 mM for the 7-TMCA acylation process and \({\text{C}}_{{{\text{KA}}}}^{{\text{o}}}\) = 140–170 mM for the 7-ACA acylation process. Under such optimal conditions, the duration of the acylation processes (the time to reach the plateau of the maximum concentration of the product) was 50 and 60 min for the synthesis of CFM and S-p CFM, respectively.
Table 3 shows the results achieved in the synthesis of CFM and S-p CFM catalyzed by IECASA under the chosen optimal conditions that provide a high degree of KA transformation into β-lactam (ηmax, %) and a high concentration of the target product in the final reaction mixture (\({\text{C}}_{{{\text{prod}}}}^{{{\text{max}}}},\) mM), which is necessary for development of an efficient process for the isolation and purification of the antibiotic. The results in this work on the kinetically controlled synthesis of CFM and S-p CFM catalyzed by IECASA are compared in Table. 3 with the best results available in the literature on the biocatalytic synthesis of these compounds [24, 25].
Comparison of IECASA-catalyzed processes for the synthesis of CFM from 7-TMCA, and S-p CFM from 7-ACA (Fig. 1, transformations 2 and 3) indicated the advantages of the second process included an increase in the yield of the acylation product from 80.8 ± 1.9 to 88.6 ± 2.0% and an increase in the concentration of the target β-lactam at the end of the process in the reaction mixture by 1.5 times. It should be noted that an increase in the concentration of the S-p CFM product in the final reaction mixture as compared to the CFM concentration was achieved due to the both an increase in the yield of the transformation product and the achievement of a higher initial 7-ACA concentration as compared to the initial 7-TMCA concentration.
Thus, the biocatalytic acylation of 7-ACA with mandelic acid methyl ester (Fig. 1, transformation 3) proceeded more efficiently than the acylation of 7-TMCA (Fig. 1, transformation 2). It is important that the chemical transformation of S-p CFM into CFM (Fig. 1, transformation 4) can be carried out without the isolation of S-p CFM from the reaction mixture [11, 25] and is preferable from an environmental point of view, since it proceeds under milder conditions than chemical transformation 7-ACA to 7-TMCA (Fig. 1, transformation 1). In this regard, the chemical-biocatalytic synthesis of CFM through the S-p CFM intermediate seems to be more promising for the development of a competitive technology for the production of this antibiotic. The similar conclution was done after comparison the chemical-biocatalytic pathways for the synthesis of another cephalosporin antibiotic, CEZ [11].
REFERENCES
Sharon, S.C., Cefamandole, in xPharm: The Comprehensive Pharmacology Reference, Enna, S.J. and Bylund, D.B., Eds., Elsevier Inc., 2008, pp. 1–5.
Schmidt, F.-R., in The Mycota X. Industrial Applications, Esser, K. and Hofrichter, M., Eds., Berlin: Springer-Verlag, 2010, vol. 5, pp. 101–121.
Srirangan, K., Orr, V., Akawi, L., Westbrook, A., Moo-Young, M., and Chou, C.P., Biotechnol. Adv., 2013, vol. 31, no. 8, pp. 1319–1332.
Volpato, G., Rodrigues, R.C., and Fernandez-Lafuente, R., Curr. Med. Chem., 2010, vol. 17, no. 32, pp. 3855–3873.
Wang Lu., Sklyarenko, A.V., Li Duanhua., Sidorenko, A.I., Zhao Chen., Li Jinjun, and Yarotsky, S.V., Bioprocess Biosyst. Eng., 2018, vol. 41, no. 12, pp. 1851–1867.
Kurochkina, V.B. and Sklyarenko, A.V., in Biotechnology: State of the Art and Prospects for Development, Zaikov, G.E., Ed., New York: Nova Science Publishers, 2008, pp. 175–204.
Sheldon, R.A. and Woodley, J.M., Chem. Rev., 2018, vol. 118, no. 2, pp. 801–838.
Rajasekar, V.W., Enz. Eng, 2016, vol. 5, no. 1, pp. 138–139.
Rodriguez-Herrera, R., Puc, L.E.C., Sobrevilla, J.M.V., Luque, D., Cardona-Felix, C.S., Aguilar-Gonzalez, C.N., and Flores-Gallegos, A.C., in Enzymes in the Pharmaceutical Industry for β-Lactam Antibiotic Production, Kuddus, M., Ed., Academic Press, 2019, vol. 36, pp. 627–643.
Sklyarenko, A.V., Eldarov, M.A., Kurochkina, V.B., and Yarotsky, S.V., Appl. Biochem. Microbiol., 2015, vol. 51, no. 6, pp. 627–640.
Sklyarenko, A.V., Groshkova, I.A., Sidorenko, A.I., and Yarotsky, S.V., Appl. Biochem. Microbiol., 2020, vol. 56, no. 5, pp. 452–464.
El'darov, M.A., Sklyarenko, A.V., Dumina, M.V., Medvedeva, N.V., Zhgun, A.A., Satarova, D.E., Sidorenko, A.I., Epremyan, A.S., and Yarotskii, S.V., Biomed. Khim., 2015, vol. 61, no. 5, pp. 646–651.
Eldarov, M.A., Sklyarenko, A.V., Mardanov, A.V., Beletsky, A.V., Zhgun, A.A., Dumina, M.V., Medvedeva, N.V., Satarova, D.E., Ravin, N.V., and Yarockii, S.V., Appl. Biochem. Microbiol., 2015, vol. 51, no. 5, pp. 505–510.
Kurochkina, V.B., Sklyarenko, A.V., Satarova, D.E., and Yarosky, S.V., Bioprocess Biosyst. Eng., 2011, vol. 34, no. 9, pp. 1103–1117.
McDonald, M.A., Bommarius, A.S., and Rousseau, R.W., Chem. Eng. Sci., 2017, vol. 165, pp. 81–88.
Santana, M., Ribeiro, M.P.A., Leite, G.A., Giordano, R.L.C., Giordano, R.C., and Mattedi, S., AIChE J., 2010, vol. 56, no. 6, pp. 1578–1583.
Kurochkina, V.B. and Sklyarenko, A.V., Antibiot. Khimioter., 2005, nos. 5–6, pp. 39–58.
Illanes, A. and Fajardo, A., Mol. Catal. B: Enzymatic, 2001, no. 11, pp. 587–595.
Illanes, A., Anjari, S., Arrieta, R., and Aguirre, C., Appl. Biochem. Biotechnol., 2002, vol. 97, no. 3, pp. 165–179.
Wei, D.Z. and Yang, L., Chem. Technol. Biotechnol., 2003, vol. 78, no. 4, pp. 431–436.
Toledo, M., Medina, V., and Illanes, A., Process Biochem., 2002, vol. 38, no. 3, pp. 351–360.
Illanes, A., Cabrera, Z., Wilson, L., and Aguirre, C., Process Biochem., 2003, vol. 39, no. 1, pp. 111–117.
Illanes, A., Anjari, M.S., Altamirano, C., and Aguirre, C., J. Mol. Catal. B: Enzymatic, 2004, vol. 30, no. 4, pp. 95–103.
Hernández-Jústiz, O., Terrenib, M., Pagani, G.J.L., Garcı, A., Guisán, J.M., and Fernández-Lafuente, R., Enzyme Microb. Technol., 1999, vol. 25, nos. 3–5, pp. 336–343.
Terreni, M., Ubiali, D., Pagani, G., Hernandez-Justiz, O., Fernandez-Lafuente, R., and Guisan, J.M., Enzyme Microb. Technol., 2005, vol. 36, nos. 5–6, pp. 672–679.
Funding
The work was carried out within the framework of State Assignment no. 593-00003-19 PR, “Fundamental and applied scientific work in the field of biotechnology.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by P. Kuchina
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sklyarenko, A.V., Groshkova, I.A., Krestyanova, I.N. et al. Alternative Synthesis of Cefamandole with Biocatalytic Acylation Catalyzed by Immobilized Cephalosporin-Acid Synthetase. Appl Biochem Microbiol 58, 251–260 (2022). https://doi.org/10.1134/S0003683822030127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683822030127