Abstract
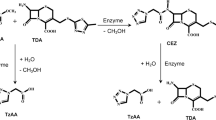
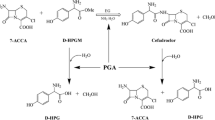
Two pathways of combined chemical and biocatalytic synthesis of the antibiotic cefazolin (CEZ) from 7-amino-cephalosporanic acid (7-ACA) with the immobilized recombinant cephalosporin-acid synthetase as the biocatalyst are compared. The first pathway involved chemical substitution with 2-mercapto-5-methylthiadiazole to modify the 3-acetoxy group in 7-ACA with subsequent biocatalytic acylation of the amino group of the product, 7-amino-3-[2-methyl-1,3,4-thiadiazol-5-yl)-thiomethyl]-3-cephem-4-carboxylic acid (TDA), with the methyl ester of 1(Н)-tetrazolylacetic acid. An alternative pathway involved biocatalytic acylation of the 7-ACA amino group to form an intermediate (S-p CEZ) that was chemically transformed into CEZ at the next step without isolation from the reaction mix. Analysis and optimization of each of the biocatalytic processes showed that 7-ACA acylation had a number of important advantages over TDA acylation with respect to the process yield, final concentration of the product in the reaction mix, and the tolerance of the process conditions with respect to enzyme activity and stability. Given the obvious environmental advantages of the process of chemical S-p CEZ transformation into CEZ over the process of TDA production from 7-ACA, we conclude that the second pathway of combined chemical and biocatalytic CEZ synthesis is preferable.







Similar content being viewed by others
REFERENCES
Nys, P.S., Kurochkina, V.B., Sklyarenko, A.V., and Veinberg, G.A., Antibiot. Khimioter., 2000, vol. 45, no. 11, pp. 36–42.
Sklyarenko, A.V., Eldarov, M.A., Kurochkina, V.B., and Yarotsky, S.V., Appl. Biochem. Microbiol., 2015, vol. 51, no. 6, pp. 627–640.
Wang, Lu, Sklyarenko, A.V., Li, Duanhua Sidorenko, A.I., Zhao, Chen., Li, Jinjun., and Yarotsky, S.V., Bioprocess Biosyst. Eng., 2018, vol. 41, no. 12, pp. 1851–1867.
US Patent no. 5387679, 1995.
Saikawa, I., Takano, S., Momonoi, K., Takakura, I., Tanaka, K., and Kutani, C., Chem. Pharm. Bull., 1985, vol. 33, no. 12, pp. 5534–5538.
Durckheimer, W., Blumbach, J., Lattrel, R., and Scheunemann, K.H., Angew. Chem., 1985, vol. 24, no. 3, pp. 180–202.
Fernandez-Lafuente, R., Guisan, J.M., Pregnolato, M., and Terreni, M., Tetrahedron Lett., 1997, vol. 38, no. 26, pp. 4693–4696.
Elander, R.P., Appl. Microbiol. Biotechnol., 2003, vol. 61, nos. 5–6, pp. 385–392.
Barber, M.S., Giesecke, U., Reichert, A., and Minas, W., Adv. Biochem. Eng. Biotechnol., 2004, vol. 88, pp. 179–215.
Schmidt, F.-R., The Mycota X. Industrial Applications, Esser, K. and Hofrichter, M., Berlin: Springer-Verlag, 2010, vol. 5, pp. 101–121.
Volpato, G., Rodrigues, R.C., and Fernandez-Lafuente, R., Curr. Med. Chem., 2010, vol. 17, no. 32, pp. 3855–3873.
Rajasekar, V.W., Enz. Eng., 2016, vol. 5, no. 1, pp. 138–139.
Rodriguez-Herrera, R., Puc, L.E.C., Sobrevilla, J.M.V., Luque, D., Cardona-Felix, C.S., Aguilar-González, C.N., and Flores-Gallegos, A.C., Enzymes in the Pharmaceutical Industry for β-Lactam Antibiotic Production, Kuddus, M., Ed., Acad. Press, 2019, ch. 36, p. 627–643.
Kurochkina, V.B. and Sklyarenko, A.V., in Enzymatic Synthesis of beta-Lactam Antibiotics, Zaikov, G.E., Ed., New York: Nova Science Publishers, 2008, pp. 175–204.
Kurochkina, V.B. and Nys, P.S., Antibiot. Khimioter., 1999, vol. 44, no. 5, pp. 12–16.
Kurochkina, V.B. and Nys, P.S., Biocatal. Biotransform., 2002, vol. 20, no. 1, pp. 35–41.
Nys, P.S. and Kurochkina, V.B., Appl. Biochem. Biotechnol., 2000, vol. 88, nos. 1–3, pp. 221–229.
RF Patent no. 2210596, 2000.
RF Patent no. 2420581, 2011.
Eldarov, M.A., Sklyarenko, A.V., Mardanov, A.V., Beletsky, A.V., Zhgun, A.A., Dumina, M.V., Medvedeva, N.V., Satarova, D.E., Ravin, N.V., and Yarockii, S.V., Appl. Biochem. Microbiol., 2015, vol. 51, no. 5, pp. 505–510.
El'darov, M.A., Sklyarenko, A.V., Dumina, M.V., Medvedeva, N.V., Zhgun, A.A., Satarova, D.E., Sidorenko, A.I., Epremyan, A.S., and Yarotskii, S.V., Biomed. Khim., 2015, vol. 61, no. 5, pp. 646–651.
Park, C.B., Lee, S.B., and Ryu, D.D., J. Mol. Catal., 2000, vol. 9, nos. 4–6, pp. 275–281.
Fernandez-Lafuente, R., Guisan, J.M., Pregnolato, M., and Terreni, M., Tetrahedron Lett., 1997, vol. 38, no. 26, pp. 4693–4696.
Hernandez-Justiz, O., Fernandez-Lafuente, R., Guisan, J.M., Negri, P., Pagani, G., and Pregnolato, M., Org. Chem., 1997, vol. 62, no. 26, pp. 9099–9106.
Berezin, I.V. and Klesov, A.A., in Prakticheskii kurs khimicheskoi i fermentativnoi kinetiki (Practical Course of Chemical and Enzymatic Kinetics), Moscow: Mosk. Gos. Univ., 1976, p. 79.
Kurochkina, V.B., Sklyarenko, A.V., Satarova, D.E., and Yarosky, S.V., Bioprocess Biosyst. Eng., 2011, vol. 34, no. 9, pp. 1103–1117.
McDonald, M.A., Bommarius, A.S., and Rousseau, R.W., Chem. Eng. Sci., 2017, vol. 165, pp. 81–88.
Bulycheva, M.S., Nys, P.S., and Savitskaya, E.M., Antibiotiki, 1977, vol. 22, no. 12, pp. 1073–1076.
Funding
This work was supported by State Project no. 595-00003-19 PR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by S. Semenova
Rights and permissions
About this article
Cite this article
Sklyarenko, A.V., Groshkova, I.A., Sidorenko, A.I. et al. Alternative Cefazolin Synthesis with a Cephalosporin-Acid Synthetase. Appl Biochem Microbiol 56, 526–537 (2020). https://doi.org/10.1134/S0003683820050130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683820050130