Abstract
Human adipose mesenchymal stem cells (haMSCs) are multipotent adult stem cells of great interest in regenerative medicine or oncology. They present spontaneous calcium oscillations related to cell cycle progression or differentiation but the correlation between these events is still unclear. Indeed, it is difficult to mimic haMSCs spontaneous calcium oscillations with chemical means. Pulsed electric fields (PEFs) can permeabilise plasma and/or organelles membranes depending on the applied pulses and therefore generate cytosolic calcium peaks by recruiting calcium from the external medium or from internal stores. We show that it is possible to mimic haMSCs spontaneous calcium oscillations (same amplitude, duration and shape) using 100 μs PEFs or 10 ns PEFs. We propose a model that explains the experimental situations reported. PEFs can therefore be a flexible tool to manipulate cytosolic calcium concentrations. This tool, that can be switched on and off instantaneously, contrary to chemicals agents, can be very useful to investigate the role of calcium oscillations in cell physiology and/or to manipulate cell fate.
Similar content being viewed by others
Introduction
Human mesenchymal stem cells (hMSCs) have the ability to differentiate into different cell types including adipocytes, chondroblasts and osteoblasts1,2,3. Human adipose-derived MSCs (haMSCs) are very similar to the bone marrow-derived ones1 but haMSCs are easier to collect making them promising candidates for cell therapy.
Even if the differentiating protocols using chemical agents to differentiate haMSC into adipocytes4,5,6,7,8, chondrocytes9,10,11 and osteocytes12,13,14 are well known, differentiation takes time (from 15 days to 1 month)4 and cannot produce all cell types. Furthermore, hMSCs spontaneously differentiate after 20 to 30 population doublings and lose their multipotency1,15,16.
HMSCs present spontaneous Ca2+ oscillations implicating (i) endoplasmic reticulum (ER) Ca2+ channels like the inositol 1,4,5-trisphosphate receptor (InsP3R) and plasma membrane (PM) Ca2+ channels as well as (ii) store-operated Ca2+ channels (SOCCs) and (iii) voltage-operated Ca2+ channels (VOCCs)16. These oscillations seem to be controlled by the Ca2+ release-recapture ER mechanisms amplified by the entry of external Ca2+ through PM Ca2+ channels16. Sun et al. reported that differentiated hMSCs present less Ca2+ oscillations than undifferentiated hMSCs and that blocking these oscillations by using a 10 V/m continuous electric field (EF) facilitates differentiation into osteogenic lineage17. Several other studies have pointed out the key role of the intracellular Ca2+ for stem cells and differentiation18. Moreover, various reports have shown that electromagnetic fields are able to influence the differentiation of stem cells by modulating the intracellular Ca2+ 19. However, the exact correlation between the intracellular calcium oscillations and the differentiation process is still unclear.
Microsecond pulsed electric field (μsPEF) of about 100 kV/m are commonly used to induce PM permeabilisation to different types of molecules (small ions21, drugs22, DNA23). The higher the EF amplitude, the greater the permeabilisation24. Since a decade, a new type of PEFs has been used: the nanosecond PEFs (nsPEFs) that are about 1 000 to 10 000 fold shorter in duration and 30 to 300 fold higher in amplitude. Application of nsPEFs can generate cytosolic Ca2+ peaks by permeabilising not only PM but also internal membranes such as the ER membranes, allowing the release of the Ca2+ stored in the ER25 to the cytosol.
The aim of this work was to develop a flexible way to manipulate cytosolic Ca2+ concentrations. This tool could be switched on and off on demand and allow to study the possibility to mimic spontaneous Ca2+ oscillations in haMSCs using nsPEFs or μsPEFs.
Results
Follow-up of the spontaneous Ca2+ oscillations in haMSCs
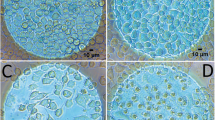
Undifferentiated haMSCs presented asynchronous spontaneous Ca2+ oscillations viewable by the Fluo-4 labelling (Fig. 1a,b). Figure 1c shows the stable repetition frequency of cytosolic Ca2+ concentration oscillations of one cell displaying 14 peaks in about 1800 s (≈128 s between each Ca2+ oscillation). Even if for each cell the rhythm of the Ca2+ oscillations was rather stable, there was a large intercellular variability in the interval between two oscillations which is in agreement with the idea of asynchronous oscillations (Fig. 1d). The mean interval between two oscillations was 82 s ± 96 s, mean ± SD (n = 160 cells).
(a) Snapshot in epifluorescence of Fluo-4 labelled haMSCs. (b) Focus on two Fluo-4 labelled cells at different times of observation. The cells presented asynchronous spontaneous oscillations. (c) Ca2+ oscillations of one haMSC extracted from a movie of 30 min (one image every 10 s). The Ca2+ oscillations displayed regular amplitude and rhythm. (d) Distribution of the oscillation periods of oscillating haMSCs. 197 cells were followed from four experiments. 175 out of 197 cells (89%) displayed oscillations and for 160 of them (81%), all along the observation period. These 160 cells were used to prepare the plot. There was a large intercellular variability. The average time between two oscillations was 82 s ± 96 s, mean ± SD and the median time was 100 s. The minimum was 26 s and the maximum was 514 s.
Generation of Ca2+ peaks using nsPEFs
Since one of our goals was to mimic the spontaneous oscillations with only one single PEF of 10 ns duration and high electric field amplitude, different magnitudes of the PEFs were tested on adherent cells with or without Ca2+ in the external medium.
In the presence of Ca2+ (Fig. 2a), a single 10 ns pulse of 7.5 MV/m was needed to induce a cytosolic Ca2+ increase showing a permeabilisation of the adherent haMSCs to Ca2+ ions. An amplitude of 16.8 MV/m was needed to induce a Ca2+ peak in 100% of the haMSCs. In this case, Ca2+ fluxes can be established across the PM and/or organelles membranes.
(a,b) Field amplitudes needed to start to permeabilise the attached haMSCs (E0) and to permeabilise all the attached haMSCs (E100) in the presence (a) or in the absence of external Ca2+ (b). E0PM and E100PM are referring to the plasma membrane whereas E0Org and E100Org are referring to the organelles membranes. These experiments have been repeated at least 4 times in the presence and in the absence of Ca2+. 17 to 30 cells per experiment were observed for each tested electric field amplitude. Lower electric field was needed to induce Ca2+ peaks in the presence of external Ca2+ than in the absence of Ca2+. (c,d) Cytosolic Ca2+peaks recorded in two different experiments with adherent haMSCs responding to seven consecutive nsPEFs amplitudes ranging from 2.7 MV.m−1 to 36 MV/m−1. The plotted cells were representative of the general cell behaviour. (c) 2 cells Ca2+ profiles from an experiment performed in the presence of external Ca2+ (complete DMEM). (d) 2 cells Ca2+ profiles from an experiment performed in the absence of external Ca2+ (S-MEM). The shape of the beginning of the Ca2+ peaks was depending on the electric field amplitude.
In the absence of external Ca2+ (Fig. 2b), 9.5 MV/m were needed to visualise a cytosolic Ca2+ increase and 21.5 MV/m were needed to observe a Ca2+ peak in 100% of the haMSCs. In that case, the mobilised Ca2+ can only be the internally stored Ca2+, revealing permeabilisation of organelles membranes.
In the presence of Ca2+, an 8.8 MV/m nsPEF induced small Ca2+ peaks whereas a 13 MV/m nsPEF induced a higher Ca2+ peak which started gradually (similar to the spontaneous oscillations). From 17 MV/m to 36 MV/m cells presented Ca2+ peaks starting with sharp rises (Fig. 2c).
In the absence of Ca2+, no effects were seen when one 10 ns nsPEF of 2.7 MV/m and 8.9 MV/m were applied (Fig. 2b,d). A nsPEF of 13 MV/m induced a small Ca2+ response. With nsPEFs from 17 to 21 MV/m Ca2+ peaks presented a slow rise at the beginning. For nsPEFs of 28 MV/m cells presented Ca2+ peaks with increasing amplitudes and sharp rises at the beginning. For the application of a 36 MV/m nsPEF, the amplitude of the responses slightly decreased (Fig. 2d). All together, these data show that the nsPEF amplitude needed to generate a Ca2+ peak was therefore lower in the presence of external Ca2+ showing that PM was more susceptible to electric pulses of 10 ns than organelles membranes.
In Fig. 3, two nsPEFs of 10 ns and of an electric field amplitude strong enough to induce Ca2+ peaks (11.3 MV/m) were applied with 200 s of interval, in order to compare the electrically induced Ca2+ peaks to the spontaneous ones. Most of the cells responded to each nsPEF by an increase in the cytosolic Ca2+ concentration. With external Ca2+, nsPEF of 11 MV/m allowed reproducing Ca2+ peaks with comparable amplitude and duration as the spontaneous ones without stopping the spontaneous oscillations (Fig. 3a). If nsPEFs were delivered during the decreasing time of the spontaneous Ca2+ peaks, cells still showed an increase in Ca2+ concentration (Fig. 3a,b). But, there was no effect if the nsPEF was delivered during the rising step of the spontaneous Ca2+ oscillations (Fig. 3c). On Fig. 3d, each nsPEF occurred between two spontaneous Ca2+ oscillations and generated a Ca2+ peak (of smaller amplitude, in this particular cell, compared to the spontaneous oscillations) without stopping the spontaneous oscillations.
47 cells were treated in this experiment where two nsPEFs of 10 ns were applied. 39 of them (83%) presented a Ca2+ peak due to the application of a nsPEF. Four of the 39 responding cells are plotted here. In all the cases, the two nsPEFs were applied at 400 s and 600 s of the recording. (a–c) Application of nsPEFs during spontaneous peaks. If the nsPEF is delivered simultaneously with the decreasing part of a spontaneous oscillation, the cell can exhibit an electrically induced Ca2+ peak (a,b). There was no electrically induced Ca2+ peak if the nsPEF was delivered during the rising part of the spontaneous oscillation (c). (d) The nsPEFs were applied between two spontaneous oscillations. The cell presented a small induced Ca2+ peak. Amplification (see Fig. 6) could occur (a) or not (d) at this electric field amplitude, depending on the cells. In all the cases the application of nsPEFs did not stop the spontaneous oscillations.
HaMSCs were also treated in suspension without external Ca2+ and they also presented Ca2+ peaks with sharp rises in response to nsPEFs due to the release of Ca2+ from the internal stores and mostly the ER (Fig. 4). The amplitude of the Ca2+ peaks induced by a train of nsPEFs decreased progressively from pulse to pulse even when nsPEFs of higher amplitudes were delivered.
Cells were pulsed in S-MEM. The cells are laid down on the bottom of the channel of the exposure device. The nsPEFs were delivered every 100 s. The 3 first arrows correspond to a 10 ns pulse of 20 MV/m. The last 2 arrows correspond to a 10 ns pulse of 24 MV/m. Each nsPEF induced an immediate and sharp rise in cytosolic Ca2+ concentration. In response to two nsPEFs of identical field strength, amplitude of the second Ca2+ peak was 10 fold smaller than the first one. Even a higher nsPEF amplitude (for example 24 MV/m) delivered after 3 nsPEFs of 20 MV/m did not generate a higher Ca2+ peak, and did not even stop the progressive decrease in Ca2+ peaks amplitude.
Generation of Ca2+ peaks using μsPEFS
Reproduction of spontaneous oscillations (same amplitude and duration) was achieved using one μsPEF of 100 μs and 15–31 kV/m. However, not all the cells responded. The higher the μsPEF amplitude, the higher the percentage of cells presenting a Ca2+ response (up to 98% with the delivery of a μsPEF of 31 kV/m, Supplementary Table S1). Interestingly, part of the responding cells presented a slow rise at the beginning of the peak (Supplementary Table S1), like the spontaneous oscillations. The other cells exhibited a sharp rise in the cytosolic Ca2+ concentration (Fig. 5). The lower the μsPEF amplitude, the higher the percentage of cells presenting a slow rise at the beginning of the Ca2+ peaks (Supplementary Table S1). The amplitudes of the induced Ca2+ peaks were similar to the amplitudes of the spontaneous Ca2+ oscillations. The μsPEF induced Ca2+ peaks produced are due to external Ca2+ entry through the PM, since no peak was detected in the absence of external Ca2+ (data not shown).
Spontaneous oscillations were not interrupted by the application of six μsPEFs (Fig. 5). The addition of 30 μM of propidium iodide (PI) during the treatment or 30 min after the last electrical stimulation showed no PI uptake (data not shown) revealing that cells were still alive 30 min after the treatment and that these pulses lead to a very weak permeabilisation.
Finally, in the absence of external Ca2+ (S-MEM) there was no increase in Ca2+ fluorescence when applying one single μsPEF of 31 kV/m (data not shown) proving that external Ca2+ was involved in the Fig. 5 data.
Discussion
PEFs provoke the transient permeabilization of the cells membranes. The extent of this transient electropermeabilisation (also termed electroporation) depends on the PEF amplitude, duration, number and repetition frequency. As a consequence, hydrophilic molecules, including Ca2+ ions, can cross the electropermeabilized membranes. We used PEFs to generate artificial Ca2+ peaks: we show that one nsPEFs or one μsPEFs can provoke a Ca2+ peak similar to the spontaneous oscillations (same amplitude and duration, depending on the electric field amplitude and on the presence or the absence of external Ca2+). The presence or absence of serum in the media did not change the effect of the pulse, (data not shown).
Different publications already presented Ca2+ release induced by nsPEFs in non-excitable cells. It was shown that 10 nsPEFs of 30 ns at 2.5 MV/m25 or a single 60 ns pulse of 2.5 to 10 MV/m26 can cause Ca2+ release from the ER. In the former case, nsPEF affected organelles without affecting PM Ca2+ channels25. For the first time, we report that in non-excitable cells showing spontaneous Ca2+ oscillations (the haMSC) a single nsPEF as short as 10 ns can cause a Ca2+ release and a Ca2+ peak that can be almost identical to one Ca2+ oscillation as discussed below. The shape of the haMSC spontaneous oscillations is in agreement with the hypothesis described by Kawano et al.16 (the oscillations are initiated by the release of Ca2+ stored in the ER and amplified by external Ca2+ entry through PM Ca2+ channels). Actually, some haMSCs were not presenting spontaneous Ca2+ oscillations during the observation time (11%, as indicated in the legend of Fig. 1). Most of the cells displayed Ca2+ oscillations of regular amplitude and rhythm, even though there was a large intercellular variability in the time between two consecutive oscillations (Fig. 1d and legend of Fig. 1). Since there is no reason for cell cycle synchronization in our cultures and since it has been shown that hMSCs display Ca2+ oscillations only during the G1/S phases of the cell cycle27, the non-oscillating cells could be the ones out of the G1 or S phases during the observation period. Interestingly, when one pulse is applied, all the exposed cells, synchronously, displayed a Ca2+ peak (Fig. 2a,b).
Ca2+ peak generation by μsPEF
Present knowledge assumes that μsPEF cannot cause the permeabilisation of organelles membranes since, with μsPEF, the cell membrane shields the cell inside and the main effect of the μsPEF is therefore at the level of the plasma membrane. In the presence of external Ca2+, the proportion of responding cells presenting induced Ca2+ peaks with the same shape as the spontaneous oscillations was depending on the μsPEF amplitude (Supplementary Table S1) which can be explained by the hypothesis that, the higher the μsPEF amplitude, the more massive the Ca2+ entry through the μsPEF-permeabilised PM. Figure 6 summarizes the different mechanisms involved in the Ca2+ peaks generation. It is remarkable that each time a Ca2+ peak was induced by a μsPEF, it had an amplitude and a duration similar to those of the spontaneous Ca2+ oscillations. According to Kawano et al.16, SOCCs and VOCCS are involved in the amplification mechanism of spontaneous Ca2+ oscillations observed in haMSCs (Fig. 6a). It is therefore possible that a Ca2+-induced Ca2+ release (CICR) pathway involving the release of calcium from the ER and activation of VOCCS and SOCCS is also involved in the amplification of the electrically induced Ca2+ peaks if the initially μsPEF-induced Ca2+ permeabilisation is large enough (Fig. 6b). The amplification through SOCCs and VOCCs seemed to be controlled by a threshold phenomenon. But, regarding to the intercellular variability in our experiments, it was not possible to determine this threshold.
(a) Spontaneous oscillations according to Kawano et al.16. (b) Ca2+ peak triggered by μsPEF in the presence of Ca2+. When a low electric field amplitude was applied (from 15 to 25 kV/m) the shape of the induced Ca2+ peaks was the same as the one of the spontaneous oscillations. When a higher electric field amplitude was applied, the amplitude and the duration were the same as the ones of spontaneous oscillations but the shape of the beginning of the peak was sharper than the one of spontaneous oscillations. With μsPEFs only the plasma membrane was permeabilized. (c) Ca2+ peak triggered by nsPEF in the absence of Ca2+. The higher the electric field strength, the higher the amplitude of the induced Ca2+ peak. (d) Ca2+ peak triggered by nsPEFs in the presence of Ca2+. At low electric field, only the plasma membrane was permeabilized. At moderate and high electric field amplitudes, the plasma membrane and the ER membrane were permeabilized. Black arrows represent Ca2+ flows. The number 1 corresponds to the event that initiates the calcium peak, the number 2 is the amplification of the calcium peak and the number 3 is the return to the basal state.
The application of low amplitude μsPEFs (15 to 25 kV/m) seems to be interesting to synchronously cause a larger proportion of cells reproducing the same shape of Ca2+ peaks as the spontaneous oscillations. At this electric field amplitude not all the cells responded (Fig. 6b “low field”). Application of higher amplitude μsPEFs (31 kV/m) mostly generated Ca2+ peaks with a sharp rise (Fig. 6b, “high field”) due to larger PM permeabilisation to Ca2+, without organelle permeabilisation. This description is in agreement with the classical models of cell electropermeabilisation24,28.
Ca2+ peak generation by nsPEF
It is known that nsPEFs can permeabilise the ER membrane25 and the PM29,30, and that the electropermeabilisation occurs at the level of the lipid bilayer (according to molecular dynamics simulations31,32 and because lipid vesicles can be permeabilised)33,34.
In the absence of external Ca2+
nsPEF amplitudes of more than 9.5 MV/m were needed to induce a Ca2+ peak and the higher the nsPEF strength, the higher the Ca2+ peak amplitude as well as the sharper the beginning of the peak (Fig. 2d): Ca2+ peaks were due to the release of internally stored Ca2+ (mostly in the ER) which is the only source of Ca2+ in this case (Fig. 6c). Considering the shape of the Ca2+ response, nsPEFs of about 17–21 MV/m induced Ca2+ responses of smaller amplitude than the spontaneous oscillations but with similar gradual increases at the beginning. For higher nsPEFs amplitudes, Ca2+ peaks were not only higher but moreover they started more sharply indicating again that, in this case, Ca2+ flowed from the internal store and no amplification occurred through SOCCs and VOCCs. Correspondingly, a depletion of the internal Ca2+ stores can explain the decrease in Ca2+ peaks amplitude in response to several nsPEFs of 20 MV/M or 24 MV/m (Fig. 4) or to the last nsPEF applied in Fig. 2d. According to Kawano et al.16,35, without external Ca2+, refilling of the calcium stores should not be possible. Furthermore, since nsPEFs also permeabilise the PM, the leakage of the cytosolic Ca2+ through the PM (included the Ca2+ released from the stores) could enhance the internal stores depletion.
In the presence of external Ca2+
Ca2+ peaks amplitudes were either lower than or as high as the ones of the spontaneous oscillations (Fig. 6d). These data are also in agreement with the amplification mechanism suggested with the μsPEFs. The fact that some cells exposed to a nsPEF of less than 11 MV/m showed Ca2+ peaks of a lower amplitude than the spontaneous oscillations (Fig. 6d 1) could be due to the fact that membranes were only slightly permeabilised. Indeed, at these field amplitudes, nsPEF-caused permeabilisation should be reduced in duration or in intensity with respect to μsPEF-caused permeabilisation (under our experimental conditions, the nsPEF are 104 times shorter than the μsPEF). For nsPEFs of less than 9,5 MV/m, only the external Ca2+ was involved. For higher nsPEF amplitudes, both the PM and the organelles membranes were permeabilised. When nsPEFs of 11 MV/m to 13 MV/m were applied, the Ca2+ increases were more important and the shape of the beginning of the peak was similar to that of spontaneous oscillations (Fig. 6d 2, “moderate field”). When nsPEFs of even higher amplitude were applied (from 17 to 40 MV/m) haMSCs presented Ca2+ peaks with sharp rising (Fig. 6d 2 “high field”). This could be explained by the fact that membranes permeabilisation is large enough to lead to a fast and massive increase in cytosolic Ca2+, rapidly amplified by the SOCCs and the VOCCs (Fig. 6d 2 “1 + 2”). The amplification mechanism might control the amplitude of these peaks which is similar to the amplitude of the peaks starting by a slow increase. Indeed, the peaks presenting a sharp rise in the Ca2+ concentration mostly reached the same maximum as the ones showing gradual increases at the beginning. Moreover, it is noteworthy that the application of nsPEF during the rising part of a spontaneous oscillation does not display any additional effect (Fig. 3c). This observation reinforces the hypothesis that, in the presence of external Ca2+, the spontaneous oscillations and the induced peaks are partly using the same mechanisms (involvement of SOCCs and VOCCs), explaining why there is no additive effect and why the amplitude of the induced Ca2+ peaks is similar to the Ca2+ oscillations amplitude.
A decade ago, it was thought that nsPEFs would only permeabilise organelles, but it has been shown that nsPEFs also permeabilise PM29. However, it is still commonly assumed that lower amplitude nsPEFs are needed to permeabilise organelles than PM because the duration of the pulse is shorter than the time necessary to charge the membrane and because the charging time of the membrane of a small organelle is shorter than the charging time of the membrane of a larger element like the entire cell. However, we show here that, with haMSCs, pulses of 10 ns duration and Ca2+ ions as permeabilization marker, the amplitude of the electric pulses necessary to permeabilise the PM is lower than the PEF amplitude necessary to permeabilize the organelles membrane. Interestingly, the comparison is made here using the same marker (Ca2+) and the same system to visualise it (Fluo-4, used under identical conditions). Our conclusions are supported by Semenov et al.36 who recently reported that.using a single longer pulse of 60 ns, it is easier to permeabilise PM than organelles in CHO cells (the permeabilisation marker was also the Ca2+).
The characteristics of the permeabilisation marker and the sensitivity of the techniques used to detect it are important parameters that must be taken into account. Indeed, we showed elsewhere37 that the notion of chemical permeabilisation strongly depends on the dye and the technique of observation used. In all the cases, haMSCs recovered in less than two minutes, meaning that Ca2+ could no longer be mobilised across the membranes. The data also suggest that induced PM permeabilisation or organelles membrane permeabilisation was fully reversible.
Whatever the mechanism for the rise in cytosolic Ca2+ concentration, the Ca2+ should be pumped back to the ER through SERCA pumps (sarco/ER Ca2+ ATPase) and/or released to the external medium through the NCX channel (Na+/Ca2+ exchanger) and PMCA (PM Ca2+ ATPase) as demonstrated by Kawano et al.35.
In conclusion, we developed new flexible tools to control internal Ca2+ concentrations on demand and to reproduce spontaneous Ca2+ oscillations in haMSCs using nsPEFs and μsPEFs. The important parameters (amplitude, duration and shape of the gradient in Ca2+ concentration) in the regulation of cell physiology by Ca2+ peaks are not known but with these new tools, different shapes of Ca2+ peaks can be produced. Interestingly, the perturbation of cell membranes is minimal and fully reversible when the electric pulses are delivered at repetition frequencies compatible with the spontaneous oscillations rates. This way to manipulate the cytosolic Ca2+ concentration presents the great advantage that it can be instantaneously switched on and off, which is not possible with chemical agents. Thus it is possible to manipulate cytosolic Ca2+ concentrations on demand by electrical manipulation and to activate or not SOCCs and VOCCs. This approach can help to understand the implication of Ca2+ oscillations in biological processes like cell differentiation and to determine the important parameters of the Ca2+ peaks in cell physiology.
Methods
Cells and culture conditions
HaMSCs were isolated from surgical waste of individuals undergoing elective lipoaspiration. Samples were obtained after written informed consent from all the donors, in accordance with France and European legislations6,38. The lipoaspirates were surgical waste and as such the French legislation (Art.L. 1245-2 du Code de la Santé Publique) establishes that the authorization from an ethics committee is not required. Cells were grown in DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 10% foetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin. Cell culture chemicals were purchased from Life Technologies (Cergy Pontoise, France). Cells were propagated at 37 °C in a humidified 5% CO2 atmosphere by passing them every 3–4 days (one passage corresponding to one doubling time of the population). HaMSCs were isolated and characterised as reported in Liew et al.39. Briefly, HaMSCs were isolated by plastic adherence and characterised by flow cytometry analysis of specific surface antigens (positive for CD105, CD90 and CD44 and negative for CD34 and CD45, purity above 90%) and by adipogenic and osteogenic differentiation. Two different media were used in the experiments: complete DMEM (containing 1.8 mM CaCl2 and 10% foetal bovine serum) and S-MEM (Suspension Minimal Essential Medium, without calcium and foetal bovine serum). Serum was not used in the buffer without calcium (SMEM) since it contains some calcium.
Electric pulses generators and exposure devices
A commercial generator purchased from FID (FID GmbH, Model FPG 10-ISM10, Burbach, Germany) was used to treat the cells. It generates trapezoidal monopolar pulses of 10 ns. The exposure devices were microchambers with parallel gold electrodes, 25 μm thick, with a gap between the electrodes of 300 μm or 150 μm. This device allows a high spatial homogeneity of the electric field (variation lower than 1.6% in z and lower than 2–10% maximum in x) as described in Dalmay et al.40.
Electric pulses of 100 μs were generated using a CliniporatorTM (Igea, Carpi, Italy) connected to two parallel stainless steel rods of 1 mm diameter, 4 mm apart that were shaped to enter a 24 plate well and used as electrodes. The whole system was installed on an inverted microscope (see below).
Imaging and images treatment
Cells were cultured on glass cover slides for at least 24 hours to obtain well spread out cells. In order to visualise the Ca2+ oscillations, the cells were incubated with 5 μM of the Ca2+ fluorescent marker, Fluo-4 AM (λex = 496 nm, λem = 515 nm) for 30 min in a humidified atmosphere with 5% CO2 at 37 °C in complete DMEM. To easily localise the cells, the incubation buffer also contained 375 nM of the nuclear fluorescent dye Hoechst 33342 (λex = 350 nm, λem = 461 nm). There is no interference between Fluo-4 and Hoechst 33342 fluorescences because their emission wavelengths are separated enough. The slides were washed three times with PBS (Phosphate Buffered Saline), turned upside down and placed on the top of the microchamber filled with complete DMEM or S-MEM (the cells being thus located inside the channel).
All the observations done with microsecond pulses exposure were done at 37 °C with 5% of CO2. Indeed, the microscope had a heating stage controlled by a Tempcontrol 37-2 digital (Pecon GmbH, Erbach, Germany) and the CO2 was maintained to 5% with a CTI-Controller 3700 digital (Pecon GmbH) into a Plexiglas chamber placed over the heating stage.
Images of haMSCs were taken every 10 s for 10 to 40 min with a Zeiss AxioCam Hrc controlled by the Axio Vision 4.6 software (Carl Zeiss, Oberkochen, Germany) on a Zeiss Axiovert S100 epifluorescence inverted microscope. The pulses were always delivered after at least 5 min of observation and 2 s before the next image. Bright field images and Fluo-4 and Hoechst 33342 fluorescence images were sequentially taken using shutters controlled by the AxioVision 4.6 software. The minimum opening time of the shutters for the fluorescent light was about 500 ms. In order to decrease the light energy applied on the cells, a 90% density Filter Thorlabs (NE110B, Maisons-Lafitte, France) was used.
The Hoechst 33342 was used to track the cells during videomicroscopy. Indeed, nuclei were recognised and the individual cells were tracked using the Cell Profiler software (Broad Institute, Cambridge, USA), allowing the automatic measurement of the Fluo-4 fluorescence of each cell on every image. Curves were plotted with a program in Matlab.
Determination of the EF amplitudes needed to permeabilise Ca2+ internal stores and PM
Different increasing field amplitudes were applied on the same cells of a coverslide as it was difficult to compare the responses on totally different cells because of the intercellular variability. On each group of cells, 7 to 8 PEFs of 10 ns were applied with an interval of 300 s. The range of field amplitudes was from 2.5 MV/m up to 40 MV/m.
Statistics plotting
On the box plot, minima and maxima are represented at the ends of the whiskers. The rectangle is defined by the median and by the first and the third quartile of the sample.
Additional Information
How to cite this article: de Menorval, M.-A. et al. Electric pulses: a flexible tool to manipulate cytosolic calcium concentrations and generate spontaneous-like calcium oscillations in mesenchymal stem cells. Sci. Rep. 6, 32331; doi: 10.1038/srep32331 (2016).
References
Lee, R. H. et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 14, 311–324, 10.1159/000080341 (2004).
Lindroos, B., Suuronen, R. & Miettinen, S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev 7, 269–291, 10.1007/s12015-010-9193-7 (2011).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317, 10.1080/14653240600855905 (2006).
Deslex, S., Negrel, R., Vannier, C., Etienne, J. & Ailhaud, G. Differentiation of human adipocyte precursors in a chemically defined serum-free medium. Int J Obes 11, 19–27 (1987).
Zuk, P. A. et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7, 211–228, 10.1089/107632701300062859 (2001).
Zuk, P. A. et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13, 4279–4295, 10.1091/mbc.E02-02-0105 (2002).
Hauner, H. et al. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest 84, 1663–1670, 10.1172/JCI114345 (1989).
Sen, A. et al. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. J Cell Biochem 81, 312–319, 10.1002/1097-4644(20010501)81:2<312::AID-JCB1046>3.0.CO;2-Q (2001).
Reinisch, A. et al. Humanized system to propagate cord blood-derived multipotent mesenchymal stromal cells for clinical application. Regen Med 2, 371–382, 10.2217/17460751.2.4.371 (2007).
Hudson, J. E. et al. A defined medium and substrate for expansion of human mesenchymal stromal cell progenitors that enriches for osteo- and chondrogenic precursors. Stem Cells Dev 20, 77–87, 10.1089/scd.2009.0497 (2011).
Bonab, M. M. et al. Aging of mesenchymal stem cell in vitro . Bmc Cell Biol 7, 10.1186/1471-2121-7-14 (2006).
Gimble, J. & Guilak, F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5, 362–369, 10.1080/14653240310003026 (2003).
Halvorsen, Y. D. et al. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng 7, 729–741, 10.1089/107632701753337681 (2001).
Halvorsen, Y. C., Wilkison, W. O. & Gimble, J. M. Adipose-derived stromal cells–their utility and potential in bone formation. Int J Obes Relat Metab Disord 24, Suppl 4, S41–S44 (2000).
Wall, M. E., Bernacki, S. H. & Loboa, E. G. Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose-derived human mesenchymal stem cells. Tissue Eng 13, 1291–1298, 10.1089/ten.2006.0275 (2007).
Kawano, S. et al. Characterization of Ca(2+) signaling pathways in human mesenchymal stem cells. Cell Calcium 32, 165–174, S0143416002001240 (2002).
Sun, S., Liu, Y., Lipsky, S. & Cho, M. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J 21, 1472–1480, 10.1096/fj.06-7153com (2007).
Tonelli, F. M. et al. Stem cells and calcium signaling. Advances in experimental medicine and biology 740, 891–916, 10.1007/978-94-007-2888-2_40 (2012).
Petecchia, L. et al. Electro-magnetic field promotes osteogenic differentiation of BM-hMSCs through a selective action on Ca(2+)-related mechanisms. Scientific reports 5, 13856, 10.1038/srep13856 (2015).
Golzio, M. et al. [Calcium and electropermeabilized cells]. Journal de la Societe de biologie 197, 301–310 (2003).
Frandsen, S. K. et al. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res 72, 1336–1341, 10.1158/0008-5472.CAN-11-3782 (2012).
Poddevin, B., Orlowski, S., Belehradek, J. Jr. & Mir, L. M. Very high cytotoxicity of bleomycin introduced into the cytosol of cells in culture. Biochemical pharmacology 42 Suppl, S67–S75 (1991).
Andre, F. M. & Mir, L. M. Nucleic acids electrotransfer in vivo: mechanisms and practical aspects. Current gene therapy 10, 267–280 (2010).
Schwan, H. P. Electrical properties of tissue and cell suspensions. Advances in biological and medical physics 5, 147–209 (1957).
Vernier, P. T. et al. Calcium bursts induced by nanosecond electric pulses. Biochem Bioph Res Co 310, 286–295, 10.1016/j.bbrc.2003.08.140 (2003).
Joshi, R. P. et al. Simulations of intracellular calcium release dynamics in response to a high-intensity, ultrashort electric pulse. Phys Rev E Stat Nonlin Soft Matter Phys 75, 041920 (2007).
Resende, R. R. et al. Influence of spontaneous calcium events on cell-cycle progression in embryonal carcinoma and adult stem cells. Biochim Biophys Acta 1803, 246–260, 10.1016/j.bbamcr.2009.11.008 (2010).
Kotnik, T., Pucihar, G. & Miklavcic, D. Induced Transmembrane Voltage and Its Correlation with Electroporation-Mediated Molecular Transport. J Membrane Biol 236, 3–13, 10.1007/s00232-010-9279-9 (2010).
Silve, A., Leray, I. & Mir, L. M. Demonstration of cell membrane permeabilization to medium-sized molecules caused by a single 10 ns electric pulse. Bioelectrochemistry, 10.1016/j.bioelechem.2011.10.002 (2011).
Vernier, P. T., Sun, Y. & Gundersen, M. A. Nanoelectropulse-driven membrane perturbation and small molecule permeabilization. Bmc Cell Biol 7, 37, 10.1186/1471-2121-7-37 (2006).
Tieleman, D. P. The molecular basis of electroporation. BMC biochemistry 5, 10, 10.1186/1471-2091-5-10 (2004).
Vernier, P. T. & Ziegler, M. J. Nanosecond field alignment of head group and water dipoles in electroporating phospholipid bilayers. The journal of physical chemistry. B 111, 12993–12996, 10.1021/jp077148q (2007).
Breton, M., Delemotte, L., Silve, A., Mir, L. M. & Tarek, M. Transport of siRNA through lipid membranes driven by nanosecond electric pulses: an experimental and computational study. Journal of the American Chemical Society 134, 13938–13941, 10.1021/ja3052365 (2012).
Mauroy, C. et al. Giant lipid vesicles under electric field pulses assessed by non invasive imaging. Bioelectrochemistry 87, 253–259, 10.1016/j.bioelechem.2012.03.008 (2012).
Kawano, S., Otsu, K., Shoji, S., Yamagata, K. & Hiraoka, M. Ca(2+) oscillations regulated by Na(+)-Ca(2+) exchanger and plasma membrane Ca(2+) pump induce fluctuations of membrane currents and potentials in human mesenchymal stem cells. Cell Calcium 34, 145–156 (2003).
Semenov, I., Xiao, S. & Pakhomov, A. G. Primary pathways of intracellular Ca(2+) mobilization by nanosecond pulsed electric field. Biochim Biophys Acta 1828, 981–989, 10.1016/j.bbamem.2012.11.032 (2013).
de Menorval, M. A., Mir, L. M., Fernandez, M. L. & Reigada, R. Effects of dimethyl sulfoxide in cholesterol-containing lipid membranes: a comparative study of experiments in silico and with cells. PloS one 7, e41733, 10.1371/journal.pone.0041733 (2012).
Guilak, F. et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol 206, 229–237, 10.1002/Jcp.20463 (2006).
Liew, A. et al. Robust, efficient and practical electrogene transfer method for human Mesenchymal Stem Cells using square electric pulses. Human gene therapy methods, 10.1089/hgtb.2012.159 (2013).
Dalmay, C., De Menorval, M. A., Francais, O., Mir, L. M. & Le Pioufle, B. A microfluidic device with removable packaging for the real time visualisation of intracellular effects of nanosecond electrical pulses on adherent cells. Lab on a chip 12, 4709–4715, 10.1039/c2lc40857k (2012).
Acknowledgements
Authors would like to thank Dr. Bassim Al Sakere for the haMSCs, Dr. Brigitte Attal-Trétout, Nelly Dorval and Thomas Schmid for the development of the exposure device and their general help, Dr. Philippe Leveque for the lending of the FID generator and the tap-off, and Delong Zhou for his contribution to the Cell Profiler module development. This work was supported by CNRS, University of Paris-Sud, Gustave Roussy, the Fondation EDF, the ITMO Cancer in the frame of the Plan Cancer 2015–2019 (project PC201517) and the ANR (Intcell-ANR-10-BLAN-916, IPSIOAT-ANR-11-BS09-0031, Memove-ANR-11-BS01-006 and Nanopulsebiochip-ANR-08-NANO-024). This work was conducted in the scope of the EBAM European Associated Laboratory (LEA).
Author information
Authors and Affiliations
Contributions
M.-A.M. data collection and assembly, data analysis and interpretation, and manuscript writing; F.M.A. conception and design of the study, data collection and interpretation and manuscript writing; A.S. Conception and design; C.D., O.F. and B.L.P. conception and design of the microchambers; L.M.M. conception and design of the study, data analysis and interpretation, writing and final approval of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
de Menorval, MA., Andre, F., Silve, A. et al. Electric pulses: a flexible tool to manipulate cytosolic calcium concentrations and generate spontaneous-like calcium oscillations in mesenchymal stem cells. Sci Rep 6, 32331 (2016). https://doi.org/10.1038/srep32331
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32331
- Springer Nature Limited
This article is cited by
-
Simulation of microfluidic intracellular delivery based on the synergy of cell squeezing and electrical field
Microfluidics and Nanofluidics (2024)
-
Pulse width and intensity effects of pulsed electric fields on cancerous and normal skin cells
Scientific Reports (2022)
-
A wide-band bio-chip for real-time optical detection of bioelectromagnetic interactions with cells
Scientific Reports (2018)
-
Electrical control of calcium oscillations in mesenchymal stem cells using microsecond pulsed electric fields
Stem Cell Research & Therapy (2017)
-
Damage-free peripheral nerve stimulation by 12-ns pulsed electric field
Scientific Reports (2017)