Abstract
Chronic obstructive pulmonary disease can cause muscle fibre transformation due to chronic intermittent hypoxia-hypercapnia (CIHH). Studies have shown that high expression of Sox6 in muscle could suppress type-I fibres through downregulating the PPARβ (peroxisome proliferator-activated receptor β)/ERRγ (oestrogen-related receptor γ)/microRNA pathway. However, whether this pathway is involved in CIHH-induced muscle fibre transformation is unknown. Electrical stimulation (ES) is an effective approach to ameliorate muscle dysfunction. Here, we explored the effects of ES on CIHH-induced muscle fibre transformation and the microRNA/Sox6 pathway. After CIHH exposure, both the soleus (SOL) and gastrocnemius (GC) muscles showed decreased type-I fibres. The PPARβ/ERRγ/mir-499&208b (PEM, for GC) and PPARβ/mir-499&208b (PM, for SOL) signalling cascades were suppressed, followed by elevated Sox6 expression. Low frequency electrical stimulation (LFES) activated the PEM/PM pathway and enhanced type-I fibre numbers through suppressing Sox6 in SOL and GC. High frequency electrical stimulation (HFES) promoted type-I fibre expression through activating the PEM pathway in GC. Although PPARβ expression and type-I fibres were suppressed in SOL after HFES, no significant change was found in mir-499&208b/Sox6 expression. These results suggest that the microRNA/Sox6 pathway is disturbed after CIHH. Both low and high frequency electrical stimulations induce muscle fibre transformation partly through regulating the microRNA/Sox6 pathway.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in humans1. The high mortality of this disease is closely related to skeletal muscle dysfunction2. The fact that pulmonary transplantation cannot fully retrieve exercise capacity indicates that skeletal muscle dysfunction may be an independent aetiology leading to low exercise performance3. Type-I fibre reduction is one of the primary disorders and is closely associated with low muscle endurance in COPD patients4. However, the mechanism regulating this process is still obscure. In this study, we used rats with chronic intermittent hypoxia-hypercapnia (CIHH) to mimic the pathological process of COPD and investigate the potential mechanism underlying the transformation of skeletal muscle fibre type. Previously, our CIHH rat model successfully mimicked the pathological process of COPD, impairing rat cognitive function5.
Myosin heavy chains (MyHCs) are important structures for muscle contraction. Because there are different isoforms of MyHCs, muscle fibres can be categorized as type-I and type-II. Type-I fibres are mitochondrial-abundant and resistant to fatigue (e.g., SOL)6. Type-II can be further divided into IIa, IIb and IIx sub-groups, which have a higher velocity of shortening than type-I fibres. Type-II fibres, especially type-IIb, also named glycolytic fibres, are very sensitive to fatigue (e.g., EDL). Type-IIa and type-IIx are also known as fast-twitch oxidative glycolytic fibres, which have a faster twitch speed and lower levels of oxidative enzymes than type-I fibres (e.g., GC). Muscle fibre transformation can be initiated by postnatal external stimuli: a slow to fast switch can be induced by disuse7 or hypoxia8, while a fast to slow switch can be induced by endurance training or low frequency electrical stimulation, for example9,10. Exercise endurance impairment occurs frequently in COPD patients and is mainly attributed to the fibre transformation from type-I to type-II11.
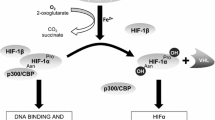
MicroRNAs are small (19–22 nt) noncoding RNAs that play an important role in diverse physiological regulation processes through repressing gene expression at the posttranscriptional 3-untranslated region (UTR)12. Mir-499 and mir-208b are muscle-specific microRNAs specifically expressed in muscles. A previous study confirmed that mir-499 and mir-208b overexpression induces the upregulation of type-I fibre numbers, partly through suppressing Sox613, a member of the Sox (Sry-related high motility group) family and a powerful slow fibre repressor, which may function as an architectural protein in transcription factor activation14. The nuclear receptor ERRγ (oestrogen-related receptor γ) is effective in the regulation of muscle mitochondrial function, muscle fibre type and revascularization15. A recent study revealed that both Myh7 (which encodes mir-208b) and Myh7b (which encodes mir-499) had putative binding sites with ERRγ and overexpression of ERRγ would lead to upregulation of mir-499 and mir-208b16. These results provided reliable evidence that mir-499 and mir-208b could be regulated by ERRγ. Meanwhile, the researchers found that overexpression of PPARβ (peroxisome proliferator-activated receptor β) could also significantly increase mir-499 and mir-208b expression, partly through activating ERRγ. A study in COPD patients showed that muscle PPARβ was significantly downregulated and was correlated with impaired muscle oxidative capacity17. Another study, focused on establishing the relationship between microRNA levels and exercise performance among COPD patients, found that decreased exercise tolerance was correlated with the downregulation of muscle mir-49918. These clues lead us to conjecture that the PPARβ/ERRγ/microRNA/Sox6 axis might be disturbed and is related to the slow fibre reduction caused by CIHH.
Electrical stimulation is a valid technique that can improve the muscle metabolic state19, endurance and power. Different electrical stimulation frequencies have different effects on muscle fibre type. Generally, LFES can induce type-I fibre expression and elevate muscle endurance20, while HFES can increase muscle strength21. Here, we sought to explore the effects of electrical stimulation on type-I fibres across two different frequencies, with a specific focus on the PPARβ/ERRγ/microRNAs/Sox6 axis in CIHH rats.
Results
Pathological changes after electrical stimulation in SOL
Hematoxylin-eosin (H & E) staining revealed (Fig. 1) many more inflammatory cells in the HFES group compared to the NC, HS, HH and LFES groups. Furthermore, in the HFES group, we found internal nuclei in some fibres and the fibrous septum was infiltrated with more inflammatory cells.
H & E staining of SOL.
These pictures show the pathological events in the SOL in each group. The scale bar represents 50 μm. The arrows indicate inflammatory cell infiltration. The triangles indicate internal nuclei. NC: normal control group; HS: hypoxia-hypercapnia+ sham stimulation group; HH: hypoxia-hypercapnia group; LFES: hypoxia-hypercapnia + low frequency electrical stimulation group; HFES: hypoxia-hypercapnia + high frequency electrical stimulation group.
Increased numbers of type-I fibres in SOL after low frequency electrical stimulation
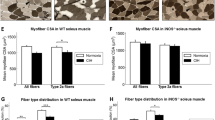
To explore the effect of electrical stimulation on SOL fibre type transformation, we applied immunochemistry to visualize the type-I fibres. The results showed (Fig. 2A) that the HS and HH groups had significantly fewer type-I fibres compared to the NC group. After a two-week low frequency electrical stimulation training, SOL type-I fibres were significantly increased compared to the HS or HH group. However, under high frequency electrical stimulation, SOL showed no significant change in type-I fibres. To further validate our results, we used western blotting and qRT-PCR (Fig. 2B,C). Western blotting corroborated the immunochemistry results. The qRT-PCR showed decreased MHC-I and increased MHC-IIa and MHC-IIx gene expression levels in the HS and HH groups compared to the NC group. In addition, significantly increased MHC-I and decreased MHC-IIa/IIx mRNA was observed in the LFES group compared to the HS and HH groups. Finally, the MHC-I mRNA level was obviously downregulated. On the other hand, MHC-IIa and MHC-IIx gene expression levels were significantly increased in the HFES group compared to the HS and HH groups. We also quantified the cross-sectional area (CSA) of type-I and type-II fibres in each group (Fig. 2D). After CIHH exposure, we found no significant change in CSA in type-I fibres compared to the NC group. However, there was a discrepancy between the LFES and HFES groups because LFES elevated the type-I and type-II fibre CSA, but HFES only increased the type-II fibre CSA.
Fibre type change of each group in SOL.
(A) Immunochemical staining for type-I fibres in SOL. Scale bar = 50 μm; (B) Western blotting for MHC-I. The optical density values are normalised to their respective tubulin loading control and the means ± SD are graphed (relative expression) to semi-quantitatively compare the protein levels; (C) Quantification of MHC-I, IIa and IIx gene expression in SOL; (D) Average cross-sectional area (CSA) of type-I and type-II muscle fibres in SOL; *P < 0.05 vs. the NC group; #P < 0.05 vs. the HS or HH group. NC: normal control group; HS: hypoxia-hypercapnia+ sham stimulation group; HH: hypoxia-hypercapnia group; LFES: hypoxia-hypercapnia+ low frequency electrical stimulation group; HFES: hypoxia-hypercapnia+ high frequency electrical stimulation group.
Electrical stimulation activates the microRNA/Sox6 pathway in SOL
Next, we analysed the signalling pathway that regulates the muscle fibre type transformation. Sox6 is a powerful type-I fibre-suppressing protein. The results showed that in the normal SOL, Sox6 expression was very low (Fig. 3A). However, a four-week chronic intermittent hypoxia-hypercapnia exposure significantly increased Sox6 expression level in the HS and HH groups compared to the NC group. In contrast, low frequency electrical stimulation resulted in a significant reduction in Sox6 expression compared to the HS and HH groups. However, there was no significant change in Sox6 level after high frequency electrical stimulation compared to the HS or HH group. Mir-499 and mir-208b could cooperatively regulate Sox6 expression in skeletal muscle and their levels were correlated with muscle fibre composition. As shown in our findings (Fig. 3B), four weeks of chronic intermittent hypoxia-hypercapnia significantly reduced both mir-499 and mir-208b expression in the HS and HH groups compared to the NC group, but levels were obviously elevated after low frequency electrical stimulation. Similarly to Sox6, there was no significant change in mir-499 and mir-208b levels in the HFES group compared to the HH and HS groups.
Electrical stimulation activated the microRNA/Sox6 pathway in SOL.
(A) Western blotting analysis of Sox6 expression. Tubulin was used as the loading control. The triangle shows the target band. (B) qRT-PCR analysis of mir-499 and mir-208b. Values are expressed as the mean ± SEM, *P < 0.05 vs. the NC group, #P < 0.05 vs. the HS or HH group. NC: normal control group; HS: hypoxia-hypercapnia + sham stimulation group; HH: hypoxia-hypercapnia group; LFES: hypoxia-hypercapnia+ low frequency electrical stimulation group; HFES: hypoxia-hypercapnia+ high frequency electrical stimulation group.
Low frequency electrical stimulation promotes PPARβ expression in SOL
We used western blotting to examine the protein levels of PPARβ and ERRγ. In our results (Fig. 4), PPARβ was enriched in normal SOL. After chronic intermittent hypoxia-hypercapnia exposure, PPARβ was lower in the HS and HH groups but was higher after low frequency electrical stimulation. On the contrary, PPARβ was significantly lower after HFES administration. There was no difference in ERRγ expression among the NC, HS and HH groups. However, the ERRγ protein level was significantly elevated after low frequency electrical stimulation administration and was significantly downregulated after high frequency electrical stimulation compared to the HS or HH group.
Low electrical stimulation promotes PPARβ expression in SOL.
Western blotting was used to establish the expression of PPARβ and ERRγ. The values are normalised to their respective tubulin loading control and the means ± SD are calculated to compare the protein levels. *P < 0.05 vs. the NC group, #P < 0.05 vs. the HS or HH group. NC: normal control group; HS: hypoxia-hypercapnia+ sham stimulation group; HH: hypoxia-hypercapnia group; LFES: hypoxia-hypercapnia +low frequency electrical stimulation group; HFES: hypoxia-hypercapnia+ high frequency electrical stimulation group.
Pathological changes after electrical stimulation in GC
We used H&E staining to investigate the pathological difference among different groups (Fig. 5). In the HS and HH groups, atrophied fibres and inflammatory cells could be found in some regions. Atrophied fibres were also found in the LFES group, but there were almost no inflammatory cells in this group. After high frequency electrical stimulation, we found many hypertrophied fibres in GC. In addition, some fibres with internal nuclei were found in this group.
H & E staining of GC.
These pictures show the pathological characteristics of GC in each group. Scale bar = 50 μm. The arrows indicate the inflammatory cells. The triangles indicate internal nuclei. NC: the normal control group; HS: the hypoxia-hypercapnia + sham stimulation group; HH: the hypoxia-hypercapnia group; LFES: hypoxia-hypercapnia + low frequency electrical stimulation group; HFES: the hypoxia-hypercapnia+ high frequency electrical stimulation group.
Increased type-I fibre in GC after electrical stimulation
Immunochemistry (Fig. 6A) revealed that type-I fibres were decreased after chronic intermittent hypoxia-hypercapnia exposure in the HS and HH groups compared to the NC group. Remarkably, low frequency electrical stimulation significantly increased the number of type-I fibres compared to the HS and HH groups. Unlike SOL, high frequency electrical stimulation also significantly elevated the type-I fibre proportion in GC compared to the HS and HH groups. To confirm this result, we applied western blotting (Fig. 6B). The results paralleled the immunochemistry results. We also studied the type-I and type-II cross-sectional areas among these groups. We found that there were no significant differences in type-I fibre CSA between groups (Fig. 6C). However, after CIHH exposure, type-II fibre CSA was significantly decreased in the HS and HH group compared to the NC group. After low frequency electrical stimulation, we did not find significant change in type-II fibre CSA, but it was obviously increased after high frequency electrical stimulation.
Type-I fibres in each group.
(A) Immunochemistry staining for type-I fibre in GC. Scale bar = 50 μm. (B) Representative Western blotting for MHC-I; the optical density values are normalised to their respective tubulin loading control and the means ± SD are graphed (relative expression) to semi-quantitatively compare the protein levels. (C) Average fibre cross-sectional area of type-I and type-II fibres. *P < 0.05 vs. the NC group; #P < 0.05 vs. the HS or HH group. NC: normal control group; HS: hypoxia-hypercapnia + sham stimulation group; HH: hypoxia-hypercapnia group; LFES: hypoxia-hypercapnia+ low frequency electrical stimulation group; HFES: hypoxia-hypercapnia+ high frequency electrical stimulation group.
Electrical stimulation reduces Sox6 expression in GC accompanied by an increase in type-I fibres
We then examined the changes in Sox6 and its suppressors mir-499 and mir-208b. As Western blotting showed (Fig. 7A), 4 weeks of chronic intermittent hypoxia-hypercapnia was associated with a significantly higher expression of Sox6. Either low frequency or high frequency electrical stimulation substantially repressed CIHH-induced Sox6 elevation in GC. We also used qRT-PCR to determine mir-499 and mir-208b expression (Fig. 7B). Levels of mir-499 and mir-208b were significantly lower in the HS and HH groups compared to the NC group. Low frequency electrical stimulation significantly upregulated mir-499 and mir-208b expression in the GC muscle fibre.
Both low and high electrical stimulation reduce Sox6 expression and induce mir-499 and mir-208b expression in GC.
(A) Western blotting analysis of Sox6 expression in GC. Tubulin was used as a loading control. (B) qRT-PCR analysis of mir-499 and mir-208b. (C) Cross-section of gastrocnemius stained for Sox6 and MHC-I by immunofluorescence. Green = MHC-I; Red = Sox6; Blue = DAPI. Scale bar = 50 μm. WG: white GC; *P < 0.05 vs. the NC group, #P < 0.05 vs. HS or the HH group;. All values represent the mean ± SEM. NC: normal control group; HS: hypoxia-hypercapnia+ sham stimulation group; HH: hypoxia-hypercapnia group; LFES: hypoxia-hypercapnia+ low frequency electrical stimulation group; HF: hypoxia-hypercapnia+ high frequency electrical stimulation.
To directly view and support our results, we performed double immunofluorescence to determine the role of Sox6 on type-I fibre expression (Fig. 7C). Consistent with previous studies, Sox6 was highly expressed in white GC (WG) where there were no type-I fibres. After 4 weeks of CIHH exposure, the Sox6 fluorescence signals were much stronger, while the type-I fibres were significantly decreased compared to the NC group. Both low frequency and high frequency electrical stimulation, however, could significantly enhance type-I fibre numbers, accompanied by the significantly reduced Sox6 fluorescence signals.
Electrical stimulation promotes PPARβ and ERRγ expression in GC from CIHH rats
We next detected PPARβ and ERRγ expression in GC (Fig. 8). PPARβ and ERRγ protein levels were significantly lower in the HS and HH groups compared to the NC group. Low frequency electrical stimulation significantly enhanced PPARβ and ERRγ levels compared to the HS and HH groups. Similar results were found after high frequency electrical stimulation, but the HFES group revealed an even higher ERRγ expression compared to the LFES group.
Electrical stimulation promotes PPARβ and ERRγ expression in GC.
Western blots for PPARβ and ERRγ are presented. Tubulin was used as a loading control. The values are expressed as the mean ± SEM. *P < 0.05 vs the NC group, #P < 0.05 vs the HB or the HH group. NC: normal control group; HS: hypoxia-hypercapnia+ sham stimulation group; HH: hypoxia-hypercapnia group; LFES: hypoxia-hypercapnia + low frequency electrical stimulation group. HFES: hypoxia-hypercapnia + high frequency electrical stimulation group.
Discussion
Reduced muscle endurance is well recognized in COPD patients and is related to exercise limitation22. Slow muscle fibre loss can lead to low muscle endurance; however, the mechanism is controversial and not well understood. Previous studies confirmed that Sox6 could reduce slow fibre numbers, but its role in the pathological conditions was less known. Herein, we show that rats under chronic intermittent hypoxia-hypercapnia exposure suffered obviously reduced type-I fibre number in the SOL and GC muscles. However, electrical stimulation can reverse this procedure partly through the derepression of Sox6 by activating the PPARβ/ERRγ/microRNA axis in GC or the PPARβ/microRNA axis in SOL. We also observed that these two muscles react differently toward high frequency electrical stimulation, indicating possibly distinctive fibre type-regulating mechanisms between them.
CIHH Exposure
After CIHH exposure, we found a significant downregulation in type-I fibres in SOL and GC. A higher percentage of type-IIa and IIx fibres indicates that the fibre transformation procedure was undertaken in both muscles, which was consistent with the findings in COPD patients11,23. Moreover, fibre cross-sectional area is an important indicator for muscle power24. A number of studies have confirmed that hypoxia could contribute to reductions in fibre CSA and interestingly, fast fibres were more susceptible to hypoxia than slow fibres25,26. In this study, we observed a significantly reduced CSA of type-II fibres in SOL and GC but no difference in type-I fibres.
Sox6 was enriched in muscles (especially in fast fibres)27 and three previous studies had found that Sox6 could act as a slow fibre suppressor during muscle development. These studies were performed in zebra fish (one study)28 and mice (two studies)29,30. However, it was still not clear whether Sox6 could also act as a slow fibre repressor in postnatal periods because the previous studies were based on gene knockout animal models. In this study, we showed a close relationship between Sox6 and type-I fibre expression. As in normal SOL, Sox6 expression was very low. Nevertheless, it was higher in GC. That difference could arise from the greater proportion of type-II fibres in GC compared to SOL. To the best of our knowledge, the relationship between Sox6 expression and hypoxia was only studied in skeletogenesis31, which revealed that hypoxia could induce Sox6 expression. Here, we observed that after exposure to chronic intermittent hypoxia-hypercapnia, contrary to the downregulation of MHC-I expression, Sox6 expression was significantly increased in SOL and GC, which might indicate that Sox6 was still functional in muscle fibre type regulation in the postnatal stage.
Mir-499 and mir-208b are highly enriched in slow muscle fibres. Previous scholars found that mir-499 could repress Sox6 expression and augment slow fibre expression in fast-twitch muscle in the zebra fish embryo32. A mir-499/208b double knockout (but not knockout separately) could significantly induce MHC-I protein downregulation13,33. It is noteworthy that Lewis18 and Donaldson34 showed that COPD patients with a lower percentage of type-I fibres had a trend towards association with lower miR-499 levels. Those findings led us to focus on the synergistic regulation of mir-499/208b in rat muscle fibre type transformation when exposed to CIHH. In our study, we found both mir-499 and mir-208b expression were significantly downregulated in SOL and GC after CIHH exposure.
PPARβ has long been thought to be an important factor in metabolic regulation35. PPARβ overexpression mice showed a significant enhancement of running capacity, correlated with an increased number of type-I muscle fibres36. How PPARβ exerts its role on skeletal muscle type transition was unknown until ERRγ was found to play a similar role in type-I fibre transition. Zhenji Gan et al.16 found that PPARβ could act as an upstream regulator to upregulate ERRγ expression. In our rat model, we showed that PPARβ was obviously decreased both in SOL and GC after CIHH exposure, while ERRγ was decreased in GC but unchanged in SOL. The downregulation of PPARβ/ERRγ/microRNA and the upregulation of Sox6 led us to speculate that the probable suppression of the PEM axis and activation of Sox6 might be partly attributed to the slow fibre reduction after CIHH exposure in GC. Although PPARβ was downregulated, ERRγ in SOL was not significantly changed compared to the NC group. This discrepancy between GC and SOL in ERRγ expression under hypoxia is still unclear. Interestingly, previous research found that neither ERRγ overexpression nor knockout would have an effect on SOL type-I fibre expression. Nevertheless, in GC, type-I fibres were significantly increased when ERRγ was overexpressed but were significantly decreased after ERRγ knockout15,37. Therefore, we concluded that ERRγ was dispensable in fibre type regulation in SOL and there might be compensatory pathways in the downregulation of mir-499 and mir-208b induced by PPARβ after CIHH exposure. To our knowledge, this is the first study demonstrating that the microRNA/Sox6 pathway is impaired in CIHH rat skeletal muscle with muscle fibre transformation.
Electrical stimulation
Exercise has been broadly used as an effective way for COPD patients to rehabilitate from skeletal muscle dysfunction38. However, some limitations prevent patients from participating in exercise training. For instance, patients with low muscle endurance and heart function may not tolerate even light training programs. It has been found, however, that an electrical stimulation (ES) program could partly mimic the exercise procedure and had a similar partial effect on muscle function. In addition, the ES program was free from those limitations. Therefore, it is suitable for muscle rehabilitation in COPD patients with poor exercise performance.
The prevalence of muscle atrophy is relatively high in COPD patients39. In our rat model, we also found numerous atrophied muscle fibres in the SOL and GC after CIHH exposure. After HFES administration, there were many hypertrophied muscle fibres in SOL and GC, which was similar to most previous studies. Interestingly, we also found that the CSA of type-II fibres in SOL and GC were selectively increased after HFES, which might indicate that the HFES program was useful to prevent muscle atrophy. It was noteworthy that after high frequency electrical stimulation, some internal nuclei were found in both SOL and GC. The meaning of the internal nuclei is controversial; some researchers believe it might be correlated with muscle hypertrophy40 and others attribute it to neuromuscular disorders41. We also observed a higher number of inflammatory cells in the SOL after HFES. From the literature, we assumed that this might partly due to the concentric contractions caused by HFES for the activation of NF-κB pathway42.
Our investigation then showed significant type-I fibre upregulation in SOL and RG after LFES. We further evaluated the potential role of the PPARβ/ERRγ/microRNA/Sox6 axis in muscle fibre regulation during this procedure. Because ERRγ might not participate in fibre type regulation in SOL, we discuss them separately. Sox6 was obviously suppressed, while both mir-499 and mir-208b were significantly upregulated in SOL and GC after LFES administration. In GC, PPARβ was significantly increased, accompanied by the increased expression of ERRγ after LFES. In SOL, PPARβ and ERRγ expression was also significantly upregulated. As there is no certain evidence that ERRγ plays a role in fibre type transformation, we presume that there might be other ways for LFES to induce mir-499/208b expression and the subsequent Sox6 suppression in SOL. In summary, we find that LFES can induce mir-499/208b expression and inhibit Sox6 expression and was related to the increase of PPARβ/ERRγ expression and type-I fibres in GC. The mechanism through which PPARβ regulating mir-499/208b expression in SOL after electrical stimulation is remained to be disentangled. To our amazement, there was also a significant increase in type-I fibres in GC after HFES, which contradicts most other data. We do not have a reasonable explanation for this discrepancy. Interestingly, a study conducted by Gondin showed that a 75 Hz neuromuscular electrical stimulation could significantly increase quadriceps muscle type-I fibre numbers; they thought the continuous activation of non-slow fibres might be a probable cause10. We then investigated the microRNA/Sox6 regulation cascades and found that PPARβ/ERRγ and mir-499/208b were significantly increased, while Sox6 was repressed in GC. These results indicated that this regulation pathway was activated and might lead to the disinhibition of slow fibre by downregulating Sox6 expression. For SOL, although PPARβ was significantly decreased after the HFES program, mir-499/208b and Sox6 were not significantly changed. The MHC-I protein level showed a trend to downregulation and the mRNA level was significantly decreased. We suppose that there might be other regulating pathways inducing slow fibre reduction after HFES treatment in SOL. For example, PPARβ downregulation might induce other slow fibre repressor activation, such as Purpβ or SP 3 43,44. Collectively, these findings revealed the potential role of microRNA/Sox6 signalling pathway in mediating the electrical stimulation induced muscle fibre transformation. And we found that the LFES program appears to particularly suited to counteract slow fibre reduction.
To summarize, the current study demonstrates that ES program is an efficient modality for improving slow fibre numbers (LFES) and fibre CSA (HFES). We also illustrates that a novel pathway regulates slow fibre expression and the role of electrical stimulation in ameliorating the slow muscle fibre reduction caused by chronic hypoxia-hypercapnia exposure. We found that LFES could effectively elevate slow fibre numbers both in SOL and GC through regulating the microRNA/Sox6 pathway. However, the HFES program could only activate this pathway in GC. This work provides new insights into understanding the role and the benefits of ES on muscle rehabilitation in COPD patients and long-term use of the ES program in improving muscle function in COPD-induced muscle dysfunction.
Methods
Ethics statement
All animal handling procedures and experimental protocols were approved by the Ethics Committee of Wenzhou Medical University (wydw2015-0057). All experiments were performed in accordance with the relevant guidelines and regulations of the Laboratory Animal Unit of Wenzhou Medical University. All efforts were made to reduce the number of animals and to minimize their suffering.
Animals
Fifty male Sprague-Dawley rats weighing 160 ± 21 g and aged 6 months were purchased from the Laboratory Animal Centre of Wenzhou Medical University. Rats were housed in the animal care facility with 12 h light/dark cycles and the house temperature was kept at approximately 23 °C. Food and water were available ad libitum.
Study design
Rats were randomly divided into five groups: the normal control group (NC, n = 10), the hypoxia-hypercapnia+ sham stimulation group (HS, n = 10), the hypoxia-hypercapnia group (HH, n = 10), the hypoxia-hypercapnia+ low frequency electrical stimulation group (LFES, n = 10) and the hypoxia-hypercapnia+ high frequency electrical stimulation group (HFES, n = 10). The chronic intermittent hypoxia-hypercapnia model was established according to Xin-long Huo et al.45. Rats in the latter four groups were raised under a low O2 gas mixture (9–11% O2 and 5–6% CO2). The exposure time was 8 h/d, 7 d/w for 4 weeks. During the latter 2 weeks, the LFES group and the HFES group underwent the electrical stimulation protocols.
Electrical stimulation protocols
The fur over the GC was carefully shaved; then, the HS, LFES and HFES groups were properly pinioned and 2 electrodes, measuring 9 cm2 (3*3 cm), were placed at the GC of both hind limbs of each rat. A portable battery-powered stimulator(HANS-200A, GENSUN MEDICAL, Nanjing, China) was used. During the training program, angular wave pulsed currents at 300 μs (pulse width) with a warm-up phase of 1 s, a stimulation phase of 2 s and a fall time of 0.5 s was applied. The work-to-rest ratio of one on and one off was delivered in both stimulation interventions. The stimulation frequency was set to 30 Hz in the LFES group and 100 Hz in the HFES group. Either the LFES or the HFES group received one training program per day and the period of each training program was 1 h. The total training program lasted 2 weeks (14 sessions). Stimulation intensity was strictly tested before the experiment and was judged by the reaction of the rats.
H&E staining
Tissues were quickly removed after chloral hydrate injection. The GC and SOL were immediately fixed with a 4% paraformaldehyde solution and paraffin-embedded. The paraffin-embedded tissue block was sectioned at 3 μm thickness on the slicing machine (Leica, Germany). The sections were stained with haematoxylin-eosin (C0105, Beyontime, China) for the demonstration of inflammation and change in fibre cross-sectional area. The muscle fibre cross-sectional area was analysed using ImageJ software.
Muscle fibre type identification
The slides were deparaffinised in 2 changes of xylene, 15 min each. The slides were rehydrated in 100% alcohol for 2 changes, 3 min each and then transferred once through 95%, 70% and 50% alcohol for 3 min each. A ten min 3% H2O2 solution incubation was used to block endogenous peroxidase activity. Then, the slides were placed into 10 mM citrate buffer and high pressure antigen retrieval was carried out to unmask the antigenic epitope. The slides were blocked by 10% goat serum for 30 min and incubated with anti-slow myosin heavy chain antibody (1:4000, Ab11083, UK) at 4 °C overnight. The slides were washed in PBS 3 times, 5 min each. Secondary antibody incubation was undertaken according to the manufacturer’s instructions (PV-9002, ZSGB, China). The slides were washed in PBS and DAB solution was applied before counterstaining the slides with haematoxylin for 7 min.
Immunofluorescent labelling of Sox6 and MHC-I
GC muscle was embedded with O.C.T. compound, frozen in liquid nitrogen-cooled isopentane and cut into 8 mm thick cryosections with a cryostat (Thermo Scientific). The sections were blocked by 10% goat serum for 30 min and then incubated with primary antibodies against Sox6 (1:1000, Ab30455, UK) and MHC-I (1:2000, Ab11083, UK) at 4 °C overnight. After washing with PBS, secondary antibodies, including goat anti-rabbit (1:200, Dylight, USA) and goat anti-mouse (1:200, Dylight, USA), were used to conjugate primary antibodies for 1 h at room temperature. After a PBS wash, the slides were stained with DAPI (1:1000, C1005, Beyotime, China) and finally mounted with Antifade Mounting Medium (P0126, Beyotime, China). Pictures were captured by the Olympus FluoView FV500 confocal microscope.
Western blotting
Equal amounts of protein (SOL: 20 μg, GC: 20 μg) were resolved in SDS-polyacrylamide gels and electrotransferred onto PVDF membranes (Millipore, USA). PVDF membranes were blocked for 2 h at room temperature with 5% non-fat dry milk. After washing in PBS, the membranes were incubated with primary antibodies against MHC-I (1:2000, Ab11083, UK), Sox6 (1:1000, Ab30455, UK), ERRγ (1:1000, Ab128930, UK), PPARβ (1:200, Santa Cruz H-74, USA), or tubulin (1:1000, Beyotime, China) at 4 °C overnight and subsequently incubated with goat anti-rabbit (1:2000, Biosharp, China) or goat anti-mouse (1:2000, Biosharp, China) secondary antibodies at room temperature for 1 h. The immunoreactive bands were detected by Western Bright Sirius (Advansta, USA).
RNA extraction and qRT-PCR analysis
Mir-499/208b transcript levels were measured using a Roche LightCycler 480 real-time PCR system (Roche Co., Germany) with the SYBR Green detection system. Total muscle DNA extraction was undertaken as follows. First, 150 mg GC and 150 mg SOL were extracted by 1 mL Trizol reagent (Invitrogen, USA). Then, 200 mL of a chloroform and isoamylol mixture (24:1) was added and mildly shaken for 30 s, followed by precipitation for 5 min and centrifugation for 10 min at 12,000 g, 4 °C. The supernatant was removed and washed with 70% alcohol twice, followed by centrifugation for 2 min and 5 min at 12000 g, 4 °C. Finally, the RNA was resolved with DEPC-H2O. The relative microRNA copy number was defined as the ratio of microRNA to U6. The primers for the mir-499 gene were mir-499-forward: 5′-GGGGTTAAGACTTGCAGTG-3′ and mir-499-reverse: 5′-CAGTGCGTGTCGTGGAGT-3′. The primers for the mir-208b gene were mir-208b-forward: 5′-GTGCAGGGTCCGAGGTATTC-3′ and mir-208b-reverse: 5′-AAGCTTTTTGCTCGCGTTATG-3′. The primers for the U6 were U6-forward: 5′- GCTTCGGCAGCACATATACTAAAAT-3′ and U6-reverse: 5′- CGCTTCACGAATTTGCGTGTCAT-3′. The primers for the MHC-I gene were MHC-I-forward: ACCCAGCAGAAGAAGACGTT and MHC-I-backward: TGCAGGTCACAATCACCACT. The primers for the MHC-IIa gene were MHC-IIa-forward: GTGCGTGAAGATGCTGTTGG and MHC-IIa-backward: CGTTTCCCGAGTCTCCTCAC. The primers for the MHC-IIx were MHC-IIx-forward: TCAGACCCACGGTCGAAGT and MHC-IIx-backward: CCACCACAAACACGGATGAC. Each real-time PCR reaction (25 μl total volume) contained cDNA (1 μl), 5×R-PCR Buffer (5.0 μl), 250 mM Mg2+ (0.3 μl), 10 mM dNTP (0.75 μl), 0.5 μl of each of the forward and reverse primers, 25×SYBR Green I (Bio-Rad, 1.0 μl), 10−3× Calibration (Bio-Rad, 1.0 μl), 5 U/μl HS-Ex-Taq (Takara, 0.25 μl) and ddH2O (14.7 μl). All data points were performed in triplicate.
Statistical Analysis
The data were analysed by 1-way ANOVA followed by a post hoc comparison test using LSD (equal variances assumed) or Dunnett’s T3 (equal variances not assumed) method when more than 2 groups were compared. The data represent the mean ± SEM, with a statistically significant difference defined as P < 0.05. Those statistical procedures were all performed with SPSS19.0.
Additional Information
How to cite this article: Huang, S. et al. Electrical stimulation influences chronic intermittent hypoxia-hypercapnia induction of muscle fibre transformation by regulating the microRNA/Sox6 pathway. Sci. Rep. 6, 26415; doi: 10.1038/srep26415 (2016).
References
Decramer, M., Janssens, W. & Miravitlles, M. Chronic obstructive pulmonary disease and comorbidities. Lancet 379, 1341–1351 (2012).
Swallow, E. B. et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 62, 115–120 (2007).
William, D.-C. M., Paul, K., John, M. & Michael, I. P. Skeletal muscle dysfunction in COPD: clinical and laboratory observations. Clin Sci (Lond) 117, 251–264 (2009).
Swallow, E. B. et al. A novel technique for nonvolitional assessment of quadriceps muscle endurance in humans. J Appl Physiol (1985) 103, 739–746 (2007).
Min, J. J. et al. Protective effect of Dl-3n-butylphthalide on learning and memory impairment induced by chronic intermittent hypoxia-hypercapnia exposure. Sci Rep 4, 5555 (2014).
Koechlin, C. et al. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med 169, 1022–1027 (2004).
Ciciliot, S. et al. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol 45, 2191–2199 (2013).
Acevedo, L. M. et al. Slow- and fast-twitch hindlimb skeletal muscle phenotypes 12 wk after (5/6) nephrectomy in Wistar rats of both sexes. Am J Physiol Renal Physiol 309, F638–647 (2015).
Short, K. R. et al. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol (1985) 99, 95–102 (2005).
Gondin, J. et al. Neuromuscular electrical stimulation training induces atypical adaptations of the human skeletal muscle phenotype: a functional and proteomic analysis. J Appl Physiol (1985) 110, 433–450 (2011).
Gosker, H. R., Zeegers, M. P., Wouters, E. F. & Schols, A. M. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax 62, 944–949 (2007).
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism and function. Cell 116, 281–297 (2004).
van Rooij, E. et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17, 662–673 (2009).
Cohen-Barak, O. et al. Cloning, characterization and chromosome mapping of the human SOX6 gene. Gene 265, 157–164 (2001).
Matsakas, A. et al. Revascularization of ischemic skeletal muscle by estrogen-related receptor-gamma. Circ Res 110, 1087–1096 (2012).
Gan, Z. et al. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J Clin Invest 123, 2564–2575 (2013).
Remels, A. H. et al. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur Respir J 30, 245–252 (2007).
Lewis, A. et al. Downregulation of the serum response factor/miR-1 axis in the quadriceps of patients with COPD. Thorax 67, 26–34 (2012).
Petrie, M., Suneja, M. & Shields, R. K. Low-frequency stimulation regulates metabolic gene expression in paralyzed muscle. J Appl Physiol (1985) 118, 723–731 (2015).
Abdellaoui, A. et al. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. Eur Respir J 38, 781–788 (2011).
Sillen, M. J. et al. Efficacy of lower-limb muscle training modalities in severely dyspnoeic individuals with COPD and quadriceps muscle weakness: results from the DICES trial. Thorax 69, 525–531 (2014).
Saey, D. et al. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 168, 425–430 (2003).
Gosker., H. R. et al. Skeletal muscle fibre-type shifting and metabolic profile in patients with chronic obstructive pulmonary disease. Eur Respir J 19, 617–625 (2002).
Seymour, J. M. et al. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax 64, 418–423 (2009).
de Theije, C. C. et al. Differential sensitivity of oxidative and glycolytic muscles to hypoxia-induced muscle atrophy. J Appl Physiol (1985) 118, 200–211 (2015).
Faucher, M. et al. Matched adaptations of electrophysiological, physiological and histological properties of skeletal muscles in response to chronic hypoxia. Pflugers Arch 450, 45–52 (2005).
Hagiwara, N. et al. Sox6 is a candidate gene for p100H myopathy, heart block and sudden neonatal death. PNAS 97, 4180–4185 (2000).
Hagiwara, N., Ma, B. & Ly, A. Slow and fast fiber isoform gene expression is systematically altered in skeletal muscle of the Sox6 mutant, p100H. Dev Dyn 234, 301–311 (2005).
Hagiwara, N., Yeh, M. & Liu, A. Sox6 is required for normal fiber type differentiation of fetal skeletal muscle in mice. Dev Dyn 236, 2062–2076 (2007).
Quiat, D. et al. Concerted regulation of myofiber-specific gene expression and muscle performance by the transcriptional repressor Sox6. PNAS 108, 10196–10201 (2011).
Amarilio, R. et al. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development 134, 3917–3928 (2007).
Wang, X. et al. Prdm1a and miR-499 act sequentially to restrict Sox6 activity to the fast-twitch muscle lineage in the zebrafish embryo. Development 138, 4399–4404 (2011).
Yeung, F. et al. Myh7b/miR-499 gene expression is transcriptionally regulated by MRFs and Eos. Nucleic Acids Res 40, 7303–7318 (2012).
Donaldson, A. et al. Increased skeletal muscle-specific microRNA in the blood of patients with COPD. Thorax 68, 1140–1149 (2013).
Luquet, S. et al. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J 17, 2299–2301 (2003).
Wang, Y. X. et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2, e294 (2004).
Narkar, V. A. et al. Exercise and PGC-1alpha-independent synchronization of type I muscle metabolism and vasculature by ERRgamma. Cell Metab 13, 283–293 (2011).
Troosters, T. et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181, 1072–1077 (2010).
Engelen, M. P. K. J. et al. Nutritional depletionin relation to respiratory and peripheral skeletal muscle function in out-patientswith COPD. Eur Respir J 7, 1793–1797 (1994).
Bruusgaard, J. C. et al. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. PNAS 107, 15111–15116 (2010).
Swash, M. & Schwartz, M. S. Implications of longitudinal muscle fibre splitting in neurogenic and myopathic disorders. J Neurol Neurosurg Psychiatry 40, 1152–1159 (1977).
McArdle, A. et al. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 280, C621–627 (2001).
Ji, J. et al. Puralpha and Purbeta collaborate with Sp3 to negatively regulate beta-myosin heavy chain gene expression during skeletal muscle inactivity. Mol Cell Biol 27, 1531–1543 (2007).
Tsika, G., Ji, J. & Tsika, R. Sp3 proteins negatively regulate beta myosin heavy chain gene expression during skeletal muscle inactivity. Mol Cell Biol 24, 10777–10791 (2004).
Huo, X. L. et al. Efficacy of lovastatin on learning and memory deficits caused by chronic intermittent hypoxia-hypercapnia: through regulation of NR2B-containing NMDA receptor-ERK pathway. PLoS One 9, e94278 (2014).
Acknowledgements
We thank the participants for their effort and dedication. We thank Lulu Pan, Xinlong Huo and Jinjin Ming for providing suggestions to revise the manuscripts. This research was supported by the Medical Science Research Foundation of Zhejiang Province grant (NO: Y205233) and by Wenzhou City Science and Technology Bureau grant (NO:Y2005A001). This study was supported by the Research Centre of the Second Affiliated Hospital of Wenzhou Medical University.
Author information
Authors and Affiliations
Contributions
S.H., J.S., X.J. and L.J. participated in the design and preparation of the research. S.H., L.J. and J.S. analysed the data. S.H. prepared the figures. S.H., P.S. and X.W. revised and edited the manuscript. X.W. approved the final version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, S., Jin, L., Shen, J. et al. Electrical stimulation influences chronic intermittent hypoxia-hypercapnia induction of muscle fibre transformation by regulating the microRNA/Sox6 pathway. Sci Rep 6, 26415 (2016). https://doi.org/10.1038/srep26415
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26415
- Springer Nature Limited
This article is cited by
-
Hypoxia treatment and resistance training alters microRNA profiling in rats skeletal muscle
Scientific Reports (2024)
-
Sox6, A Potential Target for MicroRNAs in Cardiometabolic Disease
Current Hypertension Reports (2022)