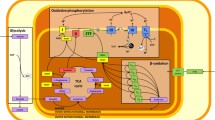
Abstract
Skeletal muscle is a metabolically active tissue and the major body protein reservoir. Drop in ambient oxygen pressure likely results in a decrease in muscle cells oxygenation, reactive oxygen species (ROS) overproduction and stabilization of the oxygen-sensitive hypoxia-inducible factor (HIF)-1α. However, skeletal muscle seems to be quite resistant to hypoxia compared to other organs, probably because it is accustomed to hypoxic episodes during physical exercise. Few studies have observed HIF-1α accumulation in skeletal muscle during ambient hypoxia probably because of its transient stabilization. Nevertheless, skeletal muscle presents adaptations to hypoxia that fit with HIF-1 activation, although the exact contribution of HIF-2, I kappa B kinase and activating transcription factors, all potentially activated by hypoxia, needs to be determined. Metabolic alterations result in the inhibition of fatty acid oxidation, while activation of anaerobic glycolysis is less evident. Hypoxia causes mitochondrial remodeling and enhanced mitophagy that ultimately lead to a decrease in ROS production, and this acclimatization in turn contributes to HIF-1α destabilization. Likewise, hypoxia has structural consequences with muscle fiber atrophy due to mTOR-dependent inhibition of protein synthesis and transient activation of proteolysis. The decrease in muscle fiber area improves oxygen diffusion into muscle cells, while inhibition of protein synthesis, an ATP-consuming process, and reduction in muscle mass decreases energy demand. Amino acids released from muscle cells may also have protective and metabolic effects. Collectively, these results demonstrate that skeletal muscle copes with the energetic challenge imposed by O2 rarefaction via metabolic optimization.





Similar content being viewed by others
References
Rolfe DF, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77:731–758
Richardson RS, Duteil S, Wary C et al (2006) Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol 571:415–424. doi:10.1113/jphysiol.2005.102327
Richardson RS, Noyszewski EA, Kendrick KF et al (1995) Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest 96:1916–1926. doi:10.1172/JCI118237
Johnson PC, Vandegriff K, Tsai AG, Intaglietta M (2005) Effect of acute hypoxia on microcirculatory and tissue oxygen levels in rat cremaster muscle. J Appl Physiol Bethesda Md 1985 98:1177–1184. doi:10.1152/japplphysiol.00591.2004
Hutter J, Habler O, Kleen M et al (1999) Effect of acute normovolemic hemodilution on distribution of blood flow and tissue oxygenation in dog skeletal muscle. J Appl Physiol Bethesda Md 1985 86:860–866
Jung F, Kessler H, Pindur G et al (1999) Intramuscular oxygen partial pressure in the healthy during exercise. Clin Hemorheol Microcirc 21:25–33
Masschelein E, Van Thienen R, D’Hulst G et al (2014) Acute environmental hypoxia induces LC3 lipidation in a genotype-dependent manner. FASEB J Off Publ Fed Am Soc Exp Biol 28:1022–1034. doi:10.1096/fj.13-239863
Richmond KN, Burnite S, Lynch RM (1997) Oxygen sensitivity of mitochondrial metabolic state in isolated skeletal and cardiac myocytes. Am J Physiol 273:C1613–C1622
Span PN, Bussink J (2015) Biology of hypoxia. Semin Nucl Med 45:101–109. doi:10.1053/j.semnuclmed.2014.10.002
Höckel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93:266–276
Semenza GL, Wang GL (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12:5447–5454
Ke Q, Costa M (2006) Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70:1469–1480. doi:10.1124/mol.106.027029
Iyer NV, Kotch LE, Agani F et al (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12:149–162
Kline DD, Peng Y-J, Manalo DJ et al (2002) Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA 99:821–826. doi:10.1073/pnas.022634199
Yu AY, Shimoda LA, Iyer NV et al (1999) Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 103:691–696. doi:10.1172/JCI5912
Yu AY, Frid MG, Shimoda LA et al (1998) Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol 275:L818–L826
Lando D, Peet DJ, Whelan DA et al (2002) Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295:858–861. doi:10.1126/science.1068592
Chandel NS, McClintock DS, Feliciano CE et al (2000) Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275:25130–25138. doi:10.1074/jbc.M001914200
Chaudhary P, Suryakumar G, Prasad R et al (2012) Chronic hypobaric hypoxia mediated skeletal muscle atrophy: role of ubiquitin-proteasome pathway and calpains. Mol Cell Biochem 364:101–113. doi:10.1007/s11010-011-1210-x
Wei W, Yu XD (2007) Hypoxia-inducible factors: crosstalk between their protein stability and protein degradation. Cancer Lett 257:145–156. doi:10.1016/j.canlet.2007.08.009
Kubis H-P, Hanke N, Scheibe RJ, Gros G (2005) Accumulation and nuclear import of HIF1 alpha during high and low oxygen concentration in skeletal muscle cells in primary culture. Biochim Biophys Acta 1745:187–195. doi:10.1016/j.bbamcr.2005.05.007
Mekhail K, Gunaratnam L, Bonicalzi M-E, Lee S (2004) HIF activation by pH-dependent nucleolar sequestration of VHL. Nat Cell Biol 6:642–647. doi:10.1038/ncb1144
Van Uden P, Kenneth NS, Rocha S (2008) Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J 412:477–484. doi:10.1042/BJ20080476
Basic VT, Jacobsen A, Sirsjö A, Abdel-Halim SM (2014) TNF stimulation induces VHL overexpression and impairs angiogenic potential in skeletal muscle myocytes. Int J Mol Med 34:228–236. doi:10.3892/ijmm.2014.1776
Aragonés J, Schneider M, Van Geyte K et al (2008) Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40:170–180. doi:10.1038/ng.2007.62
Ameln H, Gustafsson T, Sundberg CJ et al (2005) Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J Off Publ Fed Am Soc Exp Biol 19:1009–1011. doi:10.1096/fj.04-2304fje
Rasbach KA, Gupta RK, Ruas JL et al (2010) PGC-1alpha regulates a HIF2alpha-dependent switch in skeletal muscle fiber types. Proc Natl Acad Sci USA 107:21866–21871. doi:10.1073/pnas.1016089107
Zhang P, Yao Q, Lu L et al (2014) Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep 6:1110–1121. doi:10.1016/j.celrep.2014.02.011
Makino Y, Kanopka A, Wilson WJ et al (2002) Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J Biol Chem 277:32405–32408. doi:10.1074/jbc.C200328200
Cummins EP, Taylor CT (2005) Hypoxia-responsive transcription factors. Pflüg Arch Eur J Physiol 450:363–371. doi:10.1007/s00424-005-1413-7
Greijer AE, van der Groep P, Kemming D et al (2005) Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). J Pathol 206:291–304. doi:10.1002/path.1778
Metzen E, Wolff M, Fandrey J, Jelkmann W (1995) Pericellular PO2 and O2 consumption in monolayer cell cultures. Respir Physiol 100:101–106
Nimker C, Kaur G, Revo A et al (2015) Ethyl 3,4-dihydroxy benzoate, a unique preconditioning agent for alleviating hypoxia-mediated oxidative damage in L6 myoblasts cells. J Physiol Sci JPS 65:77–87. doi:10.1007/s12576-014-0348-1
Bagnall J, Leedale J, Taylor SE et al (2014) Tight control of hypoxia-inducible factor-α transient dynamics is essential for cell survival in hypoxia. J Biol Chem 289:5549–5564. doi:10.1074/jbc.M113.500405
Lindholm ME, Fischer H, Poellinger L et al (2014) Negative regulation of HIF in skeletal muscle of elite endurance athletes: a tentative mechanism promoting oxidative metabolism. Am J Physiol Regul Integr Comp Physiol 307:R248–R255. doi:10.1152/ajpregu.00036.2013
Basic VT, Tadele E, Elmabsout AA et al (2012) Exposure to cigarette smoke induces overexpression of von Hippel-Lindau tumor suppressor in mouse skeletal muscle. Am J Physiol Lung Cell Mol Physiol 303:L519–L527. doi:10.1152/ajplung.00007.2012
Stroka DM, Burkhardt T, Desbaillets I et al (2001) HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J Off Publ Fed Am Soc Exp Biol 15:2445–2453. doi:10.1096/fj.01-0125com
Pirkmajer S, Filipovic D, Mars T et al (2010) HIF-1alpha response to hypoxia is functionally separated from the glucocorticoid stress response in the in vitro regenerating human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 299:R1693–R1700. doi:10.1152/ajpregu.00133.2010
Badger JL, Byrne ML, Veraitch FS et al (2012) Hypoxic culture of human pluripotent stem cell lines is permissible using mouse embryonic fibroblasts. Regen Med 7:675–683. doi:10.2217/rme.12.55
Hagström L, Agbulut O, El-Hasnaoui-Saadani R et al (2010) Epo is relevant neither for microvascular formation nor for the new formation and maintenance of mice skeletal muscle fibres in both normoxia and hypoxia. J Biomed Biotechnol 2010:137817. doi:10.1155/2010/137817
Tsui AKY, Marsden PA, Mazer CD et al (2014) Differential HIF and NOS responses to acute anemia: defining organ-specific hemoglobin thresholds for tissue hypoxia. Am J Physiol Regul Integr Comp Physiol 307:R13–R25. doi:10.1152/ajpregu.00411.2013
Rupp T, Leti T, Jubeau M et al (2013) Tissue deoxygenation kinetics induced by prolonged hypoxic exposure in healthy humans at rest. J Biomed Opt 18:095002. doi:10.1117/1.JBO.18.9.095002
Lunde IG, Anton SL, Bruusgaard JC et al (2011) Hypoxia inducible factor 1 links fast-patterned muscle activity and fast muscle phenotype in rats. J Physiol 589:1443–1454. doi:10.1113/jphysiol.2010.202762
Viganò A, Ripamonti M, De Palma S et al (2008) Proteins modulation in human skeletal muscle in the early phase of adaptation to hypobaric hypoxia. Proteomics 8:4668–4679. doi:10.1002/pmic.200800232
Lundby C, Calbet JAL, Robach P (2009) The response of human skeletal muscle tissue to hypoxia. Cell Mol Life Sci CMLS 66:3615–3623. doi:10.1007/s00018-009-0146-8
De Palma S, Ripamonti M, Vigano A et al (2007) Metabolic modulation induced by chronic hypoxia in rats using a comparative proteomic analysis of skeletal muscle tissue. J Proteome Res 6:1974–1984. doi:10.1021/pr060614o
Pisani DF, Dechesne CA (2005) Skeletal muscle HIF-1alpha expression is dependent on muscle fiber type. J Gen Physiol 126:173–178. doi:10.1085/jgp.200509265
Ahmetov II, Hakimullina AM, Lyubaeva EV et al (2008) Effect of HIF1A gene polymorphism on human muscle performance. Bull Exp Biol Med 146:351–353
Semenza GL (2001) HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107:1–3
Horscroft JA, Murray AJ (2014) Skeletal muscle energy metabolism in environmental hypoxia: climbing towards consensus. Extreme Physiol Med. doi:10.1186/2046-7648-3-19
Ou LC, Leiter JC (2004) Effects of exposure to a simulated altitude of 5500 m on energy metabolic pathways in rats. Respir Physiol Neurobiol 141:59–71. doi:10.1016/j.resp.2004.04.001
Kim J, Tchernyshyov I, Semenza GL, Dang CV (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3:177–185. doi:10.1016/j.cmet.2006.02.002
Le Moine CMR, Morash AJ, McClelland GB (2011) Changes in HIF-1α protein, pyruvate dehydrogenase phosphorylation, and activity with exercise in acute and chronic hypoxia. Am J Physiol Regul Integr Comp Physiol 301:R1098–R1104. doi:10.1152/ajpregu.00070.2011
Brooks GA, Wolfel EE, Butterfield GE et al (1998) Poor relationship between arterial [lactate] and leg net release during exercise at 4300 m altitude. Am J Physiol 275:R1192–R1201
Green HJ, Sutton JR, Wolfel EE et al (1992) Altitude acclimatization and energy metabolic adaptations in skeletal muscle during exercise. J Appl Physiol Bethesda Md 1985 73:2701–2708
Ullah MS, Davies AJ, Halestrap AP (2006) The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 281:9030–9037. doi:10.1074/jbc.M511397200
Juel C, Lundby C, Sander M et al (2003) Human skeletal muscle and erythrocyte proteins involved in acid-base homeostasis: adaptations to chronic hypoxia. J Physiol 548:639–648. doi:10.1113/jphysiol.2002.035899
McClelland GB, Brooks GA (2002) Changes in MCT 1, MCT 4, and LDH expression are tissue specific in rats after long-term hypobaric hypoxia. J Appl Physiol Bethesda Md 1985 92:1573–1584. doi:10.1152/japplphysiol.01069.2001
Py G, Eydoux N, Lambert K et al (2005) Role of hypoxia-induced anorexia and right ventricular hypertrophy on lactate transport and MCT expression in rat muscle. Metabolism 54:634–644. doi:10.1016/j.metabol.2004.12.007
Mason SD, Rundqvist H, Papandreou I et al (2007) HIF-1alpha in endurance training: suppression of oxidative metabolism. Am J Physiol Regul Integr Comp Physiol 293:R2059–R2069. doi:10.1152/ajpregu.00335.2007
Belanger AJ, Luo Z, Vincent KA et al (2007) Hypoxia-inducible factor 1 mediates hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of peroxisome proliferator-activated receptor alpha/retinoid X receptor. Biochem Biophys Res Commun 364:567–572. doi:10.1016/j.bbrc.2007.10.062
Huss JM, Levy FH, Kelly DP (2001) Hypoxia inhibits the peroxisome proliferator-activated receptor alpha/retinoid X receptor gene regulatory pathway in cardiac myocytes: a mechanism for O2-dependent modulation of mitochondrial fatty acid oxidation. J Biol Chem 276:27605–27612. doi:10.1074/jbc.M100277200
Regnault TRH, Zhao L, Chiu JSS et al (2010) Peroxisome proliferator-activated receptor -β/δ, -γ agonists and resveratrol modulate hypoxia induced changes in nuclear receptor activators of muscle oxidative metabolism. PPAR Res 2010:129173. doi:10.1155/2010/129173
Slot IGM, Schols AMWJ, Vosse BAH et al (2014) Hypoxia differentially regulates muscle oxidative fiber type and metabolism in a HIF-1α-dependent manner. Cell Signal 26:1837–1845. doi:10.1016/j.cellsig.2014.04.016
Chaillou T, Koulmann N, Meunier A et al (2013) Effect of hypoxia exposure on the phenotypic adaptation in remodelling skeletal muscle submitted to functional overload. Acta Physiol Oxf Engl 209:272–282. doi:10.1111/apha.12110
Dutta A, Vats P, Singh VK et al (2009) Impairment of mitochondrial beta-oxidation in rats under cold-hypoxic environment. Int J Biometeorol 53:397–407. doi:10.1007/s00484-009-0224-5
Morash AJ, Kotwica AO, Murray AJ (2013) Tissue-specific changes in fatty acid oxidation in hypoxic heart and skeletal muscle. Am J Physiol Regul Integr Comp Physiol 305:R534–R541. doi:10.1152/ajpregu.00510.2012
Hoppeler H, Kleinert E, Schlegel C et al (1990) Morphological adaptations of human skeletal muscle to chronic hypoxia. Int J Sports Med 11(Suppl 1):S3–S9. doi:10.1055/s-2007-1024846
Levett DZ, Radford EJ, Menassa DA et al (2012) Acclimatization of skeletal muscle mitochondria to high-altitude hypoxia during an ascent of Everest. FASEB J Off Publ Fed Am Soc Exp Biol 26:1431–1441. doi:10.1096/fj.11-197772
Hoppeler H, Vogt M (2001) Muscle tissue adaptations to hypoxia. J Exp Biol 204:3133–3139
Zhang H, Bosch-Marce M, Shimoda LA et al (2008) Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283:10892–10903. doi:10.1074/jbc.M800102200
Band M, Joel A, Hernandez A, Avivi A (2009) Hypoxia-induced BNIP3 expression and mitophagy: in vivo comparison of the rat and the hypoxia-tolerant mole rat, Spalax ehrenbergi. FASEB J Off Publ Fed Am Soc Exp Biol 23:2327–2335. doi:10.1096/fj.08-122978
Gamboa JL, Garcia-Cazarin ML, Andrade FH (2011) Chronic hypoxia increases insulin-stimulated glucose uptake in mouse soleus muscle. Am J Physiol Regul Integr Comp Physiol 300:R85–R91. doi:10.1152/ajpregu.00078.2010
De Theije CC, Langen RCJ, Lamers WH et al (2013) Distinct responses of protein turnover regulatory pathways in hypoxia- and semistarvation-induced muscle atrophy. Am J Physiol Lung Cell Mol Physiol 305:L82–L91. doi:10.1152/ajplung.00354.2012
Bellot G, Garcia-Medina R, Gounon P et al (2009) Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29:2570–2581. doi:10.1128/MCB.00166-09
Chen R, Dioum EM, Hogg RT et al (2011) Hypoxia increases sirtuin 1 expression in a hypoxia-inducible factor-dependent manner. J Biol Chem 286:13869–13878. doi:10.1074/jbc.M110.175414
Kume S, Uzu T, Horiike K et al (2010) Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 120:1043–1055. doi:10.1172/JCI41376
Lokireddy S, Wijesoma IW, Teng S et al (2012) The ubiquitin ligase Mul1 induces mitophagy in skeletal muscle in response to muscle-wasting stimuli. Cell Metab 16:613–624. doi:10.1016/j.cmet.2012.10.005
Mason SD, Howlett RA, Kim MJ et al (2004) Loss of skeletal muscle HIF-1alpha results in altered exercise endurance. PLoS Biol 2:e288. doi:10.1371/journal.pbio.0020288
Fukuda R, Zhang H, Kim J et al (2007) HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129:111–122. doi:10.1016/j.cell.2007.01.047
Bo H, Wang Y-H, Li H-Y et al (2008) Endurance training attenuates the bioenergetics alterations of rat skeletal muscle mitochondria submitted to acute hypoxia: role of ROS and UCP3. Sheng Li Xue Bao 60:767–776
Lu Z, Sack MN (2008) ATF-1 is a hypoxia-responsive transcriptional activator of skeletal muscle mitochondrial-uncoupling protein 3. J Biol Chem 283:23410–23418. doi:10.1074/jbc.M801236200
Cicchillitti L, Di Stefano V, Isaia E et al (2012) Hypoxia-inducible factor 1-α induces miR-210 in normoxic differentiating myoblasts. J Biol Chem 287:44761–44771. doi:10.1074/jbc.M112.421255
Gelfi C, De Palma S, Ripamonti M et al (2004) New aspects of altitude adaptation in Tibetans: a proteomic approach. FASEB J Off Publ Fed Am Soc Exp Biol 18:612–614. doi:10.1096/fj.03-1077fje
Saxena S, Shukla D, Saxena S et al (2010) Hypoxia preconditioning by cobalt chloride enhances endurance performance and protects skeletal muscles from exercise-induced oxidative damage in rats. Acta Physiol Oxf Engl 200:249–263. doi:10.1111/j.1748-1716.2010.02136.x
Galbès O, Goret L, Caillaud C et al (2008) Combined effects of hypoxia and endurance training on lipid metabolism in rat skeletal muscle. Acta Physiol Oxf Engl 193:163–173. doi:10.1111/j.1748-1716.2007.01794.x
Gamboa JL, Andrade FH (2012) Muscle endurance and mitochondrial function after chronic normobaric hypoxia: contrast of respiratory and limb muscles. Pflüg Arch Eur J Physiol 463:327–338. doi:10.1007/s00424-011-1057-8
Magalhães J, Ascensão A, Soares JMC et al (2005) Acute and severe hypobaric hypoxia increases oxidative stress and impairs mitochondrial function in mouse skeletal muscle. J Appl Physiol Bethesda Md 1985 99:1247–1253. doi:10.1152/japplphysiol.01324.2004
Jacobs RA, Boushel R, Wright-Paradis C et al (2013) Mitochondrial function in human skeletal muscle following high-altitude exposure. Exp Physiol 98:245–255. doi:10.1113/expphysiol.2012.066092
Bigard AX, Brunet A, Guezennec CY, Monod H (1991) Effects of chronic hypoxia and endurance training on muscle capillarity in rats. Pflüg Arch Eur J Physiol 419:225–229
Green HJ, Sutton JR, Cymerman A et al (1989) Operation Everest II: adaptations in human skeletal muscle. J Appl Physiol Bethesda Md 1985 66:2454–2461
Snyder GK, Farrelly C, Coelho JR (1992) Adaptations in skeletal muscle capillarity following changes in oxygen supply and changes in oxygen demands. Eur J Appl Physiol 65:158–163
Niemi H, Honkonen K, Korpisalo P et al (2014) HIF-1α and HIF-2α induce angiogenesis and improve muscle energy recovery. Eur J Clin Invest 44:989–999. doi:10.1111/eci.12333
Olfert IM, Breen EC, Mathieu-Costello O, Wagner PD (2001) Skeletal muscle capillarity and angiogenic mRNA levels after exercise training in normoxia and chronic hypoxia. J Appl Physiol Bethesda Md 1985 91:1176–1184
Lundby C, Pilegaard H, Andersen JL et al (2004) Acclimatization to 4100 m does not change capillary density or mRNA expression of potential angiogenesis regulatory factors in human skeletal muscle. J Exp Biol 207:3865–3871. doi:10.1242/jeb.01225
MacDougall JD, Green HJ, Sutton JR et al (1991) Operation Everest II: structural adaptations in skeletal muscle in response to extreme simulated altitude. Acta Physiol Scand 142:421–427. doi:10.1111/j.1748-1716.1991.tb09176.x
Sillau AH, Aquin L, Bui MV, Banchero N (1980) Chronic hypoxia does not affect guinea pig skeletal muscle capillarity. Pflüg Arch Eur J Physiol 386:39–45
Hoppeler H, Vogt M, Weibel ER, Flück M (2003) Response of skeletal muscle mitochondria to hypoxia. Exp Physiol 88:109–119
Kayser B, Narici M, Binzoni T et al (1994) Fatigue and exhaustion in chronic hypobaric hypoxia: influence of exercising muscle mass. J Appl Physiol Bethesda Md 1985 76:634–640
Bigard AX, Douce P, Merino D et al (1996) Changes in dietary protein intake fail to prevent decrease in muscle growth induced by severe hypoxia in rats. J Appl Physiol Bethesda Md 1985 80:208–215
Favier FB, Costes F, Defour A et al (2010) Downregulation of Akt/mammalian target of rapamycin pathway in skeletal muscle is associated with increased REDD1 expression in response to chronic hypoxia. Am J Physiol Regul Integr Comp Physiol 298:R1659–R1666. doi:10.1152/ajpregu.00550.2009
al-Amood WS, Lewis DM (1989) A comparison of the effects of denervation on the mechanical properties of rat and guinea-pig skeletal muscle. J Physiol 414:1–16
Nilwik R, Snijders T, Leenders M et al (2013) The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48:492–498. doi:10.1016/j.exger.2013.02.012
Shimizu N, Yoshikawa N, Ito N et al (2011) Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab 13:170–182. doi:10.1016/j.cmet.2011.01.001
Pistilli EE, Bogdanovich S, Mosqueira M et al (2010) Pretreatment with a soluble activin type IIB receptor/Fc fusion protein improves hypoxia-induced muscle dysfunction. Am J Physiol Regul Integr Comp Physiol 298:R96–R103. doi:10.1152/ajpregu.00138.2009
De Theije CC, Langen RCJ, Lamers WH et al (2014) Differential sensitivity of oxidative and glycolytic muscles to hypoxia-induced muscle atrophy. J Appl Physiol Bethesda Md 1985. doi:10.1152/japplphysiol.00624.2014
Hoppeler H (1986) Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med 7:187–204. doi:10.1055/s-2008-1025758
Arthur PG, Giles JJ, Wakeford CM (2000) Protein synthesis during oxygen conformance and severe hypoxia in the mouse muscle cell line C2C12. Biochim Biophys Acta 1475:83–89
Preedy VR, Smith DM, Sugden PH (1985) The effects of 6 hours of hypoxia on protein synthesis in rat tissues in vivo and in vitro. Biochem J 228:179–185
Holm L, Haslund ML, Robach P et al (2010) Skeletal muscle myofibrillar and sarcoplasmic protein synthesis rates are affected differently by altitude-induced hypoxia in native lowlanders. PLoS One 5:e15606. doi:10.1371/journal.pone.0015606
Imoberdorf R, Garlick PJ, McNurlan MA et al (2006) Skeletal muscle protein synthesis after active or passive ascent to high altitude. Med Sci Sports Exerc 38:1082–1087. doi:10.1249/01.mss.0000222836.66391.35
Etheridge T, Atherton PJ, Wilkinson D et al (2011) Effects of hypoxia on muscle protein synthesis and anabolic signaling at rest and in response to acute resistance exercise. Am J Physiol Endocrinol Metab 301:E697–E702. doi:10.1152/ajpendo.00276.2011
Bodine SC, Stitt TN, Gonzalez M et al (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019. doi:10.1038/ncb1101-1014
Rommel C, Bodine SC, Clarke BA et al (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3:1009–1013. doi:10.1038/ncb1101-1009
Costes F, Gosker HR, Féasson L et al (2015) Impaired exercise training-induced muscle fiber hypertrophy and Akt/mTOR pathway activation in hypoxemic COPD patients. J Appl Physiol Bethesda Md 1985. doi:10.1152/japplphysiol.00557.2014
Amirouche A, Durieux A-C, Banzet S et al (2009) Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology 150:286–294. doi:10.1210/en.2008-0959
Trendelenburg AU, Meyer A, Rohner D et al (2009) Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296:C1258–C1270. doi:10.1152/ajpcell.00105.2009
Chaillou T, Koulmann N, Simler N et al (2012) Hypoxia transiently affects skeletal muscle hypertrophy in a functional overload model. Am J Physiol Regul Integr Comp Physiol 302:R643–R654. doi:10.1152/ajpregu.00262.2011
Hayot M, Rodriguez J, Vernus B et al (2011) Myostatin up-regulation is associated with the skeletal muscle response to hypoxic stimuli. Mol Cell Endocrinol 332:38–47. doi:10.1016/j.mce.2010.09.008
Van den Borst B, Schols AMWJ, de Theije C et al (2013) Characterization of the inflammatory and metabolic profile of adipose tissue in a mouse model of chronic hypoxia. J Appl Physiol Bethesda Md 1985 114:1619–1628. doi:10.1152/japplphysiol.00460.2012
Sanchez AMJ, Csibi A, Raibon A et al (2012) AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J Cell Biochem 113:695–710. doi:10.1002/jcb.23399
Wadley GD, Lee-Young RS, Canny BJ et al (2006) Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am J Physiol Endocrinol Metab 290:E694–E702. doi:10.1152/ajpendo.00464.2005
Chaillou T, Koulmann N, Meunier A et al (2014) Ambient hypoxia enhances the loss of muscle mass after extensive injury. Pflüg Arch Eur J Physiol 466:587–598. doi:10.1007/s00424-013-1336-7
Britto FA, Begue G, Rossano B et al (2014) REDD1 deletion prevents dexamethasone-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab 307:E983–E993. doi:10.1152/ajpendo.00234.2014
D’Hulst G, Jamart C, Van Thienen R et al (2013) Effect of acute environmental hypoxia on protein metabolism in human skeletal muscle. Acta Physiol Oxf Engl 208:251–264. doi:10.1111/apha.12086
Horak P, Crawford AR, Vadysirisack DD et al (2010) Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc Natl Acad Sci USA 107:4675–4680. doi:10.1073/pnas.0907705107
Li Y, Wang Y, Kim E et al (2007) Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J Biol Chem 282:35803–35813. doi:10.1074/jbc.M705231200
Koumenis C, Naczki C, Koritzinsky M et al (2002) Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol 22:7405–7416
Bodine SC, Baehr LM (2014) Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab 307:E469–E484. doi:10.1152/ajpendo.00204.2014
Gille T, Randrianarison-Pellan N, Goolaerts A et al (2014) Hypoxia-induced inhibition of epithelial Na(+) channels in the lung. Role of Nedd4-2 and the ubiquitin-proteasome pathway. Am J Respir Cell Mol Biol 50:526–537. doi:10.1165/rcmb.2012-0518OC
Köditz J, Nesper J, Wottawa M et al (2007) Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood 110:3610–3617. doi:10.1182/blood-2007-06-094441
Elvidge GP, Glenny L, Appelhoff RJ et al (2006) Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1alpha, HIF-2alpha, and other pathways. J Biol Chem 281:15215–15226. doi:10.1074/jbc.M511408200
Cummins EP, Berra E, Comerford KM et al (2006) Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA 103:18154–18159. doi:10.1073/pnas.0602235103
Ebert SM, Dyle MC, Kunkel SD et al (2012) Stress-induced skeletal muscle Gadd45a expression reprograms myonuclei and causes muscle atrophy. J Biol Chem 287:27290–27301. doi:10.1074/jbc.M112.374777
Fox DK, Ebert SM, Bongers KS et al (2014) p53 and ATF4 mediate distinct and additive pathways to skeletal muscle atrophy during limb immobilization. Am J Physiol Endocrinol Metab 307:E245–E261. doi:10.1152/ajpendo.00010.2014
Cai D, Frantz JD, Tawa NE et al (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119:285–298. doi:10.1016/j.cell.2004.09.027
Murray AJ, Montgomery HE (2014) How wasting is saving: weight loss at altitude might result from an evolutionary adaptation. Bioessays News Rev Mol Cell Dev Biol 36:721–729. doi:10.1002/bies.201400042
Uchida T, Rossignol F, Matthay MA et al (2004) Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: implication of natural antisense HIF-1alpha. J Biol Chem 279:14871–14878. doi:10.1074/jbc.M400461200
Rane S, He M, Sayed D et al (2009) Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res 104:879–886. doi:10.1161/CIRCRESAHA.108.193102
Girard O, Koehle MS, MacInnis MJ et al (2012) Comments on point: counterpoint: hypobaric hypoxia induces/does not induce different responses from normobaric hypoxia. J Appl Physiol Bethesda Md 1985 112:1788–1794. doi:10.1152/japplphysiol.00356.2012
Mizuno M, Juel C, Bro-Rasmussen T et al (1990) Limb skeletal muscle adaptation in athletes after training at altitude. J Appl Physiol Bethesda Md 1985 68:496–502
Abdelmalki A, Fimbel S, Mayet-Sornay MH et al (1996) Aerobic capacity and skeletal muscle properties of normoxic and hypoxic rats in response to training. Pflüg Arch Eur J Physiol 431:671–679
Bigard AX, Brunet A, Guezennec CY, Monod H (1991) Skeletal muscle changes after endurance training at high altitude. J Appl Physiol Bethesda Md 1985 71:2114–2121
Young AJ, Evans WJ, Fisher EC et al (1984) Skeletal muscle metabolism of sea-level natives following short-term high-altitude residence. Eur J Appl Physiol 52:463–466
Kennedy SL, Stanley WC, Panchal AR, Mazzeo RS (2001) Alterations in enzymes involved in fat metabolism after acute and chronic altitude exposure. J Appl Physiol Bethesda Md 1985 90:17–22
Dill RP, Chadan SG, Li C, Parkhouse WS (2001) Aging and glucose transporter plasticity in response to hypobaric hypoxia. Mech Ageing Dev 122:533–545
Howald H, Hoppeler H (2003) Performing at extreme altitude: muscle cellular and subcellular adaptations. Eur J Appl Physiol 90:360–364. doi:10.1007/s00421-003-0872-9
Ripamonti M, Viganò A, Moriggi M et al (2006) Cytochrome c oxidase expression in chronic and intermittent hypoxia rat gastrocnemius muscle quantitated by CE. Electrophoresis 27:3897–3903. doi:10.1002/elps.200600104
Daneshrad Z, Garcia-Riera MP, Verdys M, Rossi A (2000) Differential responses to chronic hypoxia and dietary restriction of aerobic capacity and enzyme levels in the rat myocardium. Mol Cell Biochem 210:159–166
Green H, Roy B, Grant S et al (2000) Human skeletal muscle exercise metabolism following an expedition to mount denali. Am J Physiol Regul Integr Comp Physiol 279:R1872–R1879
Bigard AX, Sanchez H, Birot O, Serrurier B (2000) Myosin heavy chain composition of skeletal muscles in young rats growing under hypobaric hypoxia conditions. J Appl Physiol Bethesda Md 1985 88:479–486
Deveci D, Marshall JM, Egginton S (2002) Chronic hypoxia induces prolonged angiogenesis in skeletal muscles of rat. Exp Physiol 87:287–291
Faucher M, Guillot C, Marqueste T et al (2005) Matched adaptations of electrophysiological, physiological, and histological properties of skeletal muscles in response to chronic hypoxia. Pflüg Arch Eur J Physiol 450:45–52. doi:10.1007/s00424-004-1370-6
Sillau AH, Banchero N (1977) Effects of hypoxia on capillary density and fiber composition in rat skeletal muscle. Pflüg Arch Eur J Physiol 370:227–232
Wüst RCI, Jaspers RT, van Heijst AF et al (2009) Region-specific adaptations in determinants of rat skeletal muscle oxygenation to chronic hypoxia. Am J Physiol Heart Circ Physiol 297:H364–H374. doi:10.1152/ajpheart.00272.2009
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Favier, F.B., Britto, F.A., Freyssenet, D.G. et al. HIF-1-driven skeletal muscle adaptations to chronic hypoxia: molecular insights into muscle physiology. Cell. Mol. Life Sci. 72, 4681–4696 (2015). https://doi.org/10.1007/s00018-015-2025-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-2025-9