Abstract
Purpose
Exposure to hypoxia has been suggested to activate multiple adaptive pathways so that muscles are better able to maintain cellular energy homeostasis. However, there is limited research regarding the tissue specificity of this response. The aim of this study was to investigate the influence of tissue specificity on mitochondrial adaptations of rat skeletal and heart muscles after 4 weeks of normobaric hypoxia (FiO2: 0.10).
Methods
Twenty male Wistar rats were randomly assigned to either normobaric hypoxia or normoxia. Mitochondrial respiration was determined in permeabilised muscle fibres from left and right ventricles, soleus and extensorum digitorum longus (EDL). Citrate synthase activity and the relative abundance of proteins associated with mitochondrial biogenesis were also analysed.
Results
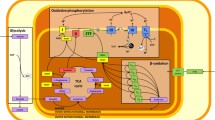
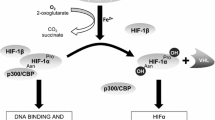
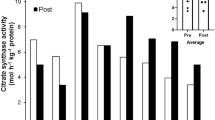
After hypoxia exposure, only the soleus and left ventricle (both predominantly oxidative) presented a greater maximal mass-specific respiration (+48 and +25%, p < 0.05) and mitochondrial-specific respiration (+75 and +28%, p < 0.05). Citrate synthase activity was higher in the EDL (0.63 ± 0.08 vs 0.41 ± 0.10 µmol min− 1 µg− 1) and lower in the soleus (0.65 ± 0.17 vs 0.87 ± 0.20 µmol min− 1 µg− 1) in hypoxia with respect to normoxia. There was a lower relative protein abundance of PGC-1α (−25%, p < 0.05) in the right ventricle and a higher relative protein abundance of PGC-1β (+43%, p < 0.05) in the left ventricle of rats exposed to hypoxia, with few differences for protein abundance in the other muscles.
Conclusion
Our results show a muscle-specific response to 4 weeks of normobaric hypoxia. Depending on fibre type, and the presence of ventricular hypertrophy, muscles respond differently to the same degree of environmental hypoxia.




Similar content being viewed by others
Data availability
All data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EDL:
-
Extensorum digitorum longus
- HIF-1α:
-
Hypoxia-inducible factor 1α
- PGC-1:
-
Peroxisome proliferator-activated receptor γ coactivator 1
- CI to CV:
-
Complex I to V
- HYP:
-
Hypoxic group
- CON:
-
Control group
- LV:
-
Left heart ventricle
- RV:
-
Right heart ventricle
- CS:
-
Citrate synthase
- BSA:
-
Bovine serum albumin
- EA:
-
Enzymatic activity
- RCR:
-
Respiratory control ratio
- SD:
-
Standard deviation
- SE:
-
Standard error
References
Bigard AX, Sanchez H, Birot O, Serrurier B (2000) Myosin heavy chain composition of skeletal muscles in young rats growing under hypobaric hypoxia conditions. J Appl Physiol 88:479–486
Bishop DJ, Thomas C, Moore-Morris T, Tonkonogi M, Sahlin K, Mercier J (2010) Sodium bicarbonate ingestion prior to training improves mitochondrial adaptations in rats. Am J Physiol Endocrinol Metab 299:E225–E233
Bloemberg D, Quadrilatero J (2012) Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7:e35273
Bortolotto SK, Stephenson DG, Stephenson GMM (1999) Fiber type population and Ca2+ activation properties of single fibers in soleus muscles from SHR and WKY rats. Am J Physiol 276:C628–C637 (Cell Physiol 45)
Britton C, Williams GR (1955) Respiratory enzymes in oxidative phosphorilation: I. Kinetics of oxygen utilisation. J Biol Chem 217:383–394
Chabert C, Bottelin P, Pison C, Dubouchaud H (2016) A low-cost system to easily measure spontaneous physical activity in rodents. J Appl Physiol 120:1907–1913
Costa LE, Boveris A, Koch OR, Taquini AC (1988) Liver and heart mitochondria in rats submitted to chronic hypobaric hypoxia. Am J Physiol Cell Physiol 255:C123–C129
Daneshrad Z, Garcia-Riera M, Verdys M, Rossi A (2000) Differential responses to chronic hypoxia and dietary restriction of aerobic capacity and enzyme levels in the rat myocardium. Mol Cell Biochem 210:159–166
Daneshrad Z, Novel-Chaté V, Birot O, Serrurier B, Sanchez H, Bigard AX, Rossi A (2001) Diet restriction plays an important role in the alteration of heart mitochondrial function following exposure of young rats to chronic hypoxia. Pflugers Arch Eur J Physiol 442:12–18
de Theije CC, Langen RCJ, Lamers WH, Schols AMWJ., Kohler SE (2013) Distinct responses of protein turnover regulatory pathways in hypoxia- and semistarvation-induced muscle atrophy. Am J Physiol Lung Cell Mol Physiol 305:L82-L91
de Theije CC, Langen RCJ, Lamers WH, Gosker HR, Schols AMWJ., Kohler SE (2015) Differential sensitivity of oxidative and glycolytic muscles to hypoxia-induced muscle atrophy. J Appl Physiol 118:200–211
Deindl E, Kolar F, Neubauer E, Vogel S, Schaper W, Ostadal B (2003) Effect of intermittent high altitude hypoxia on gene expression in rat heart and lung. Physiol Res 52:147–158
Desplanches D, Amami M, Dupré-AUcouturier S, Valdivieso P, Schmutz S, Mueller M, Hoppeler H, Kreis R, Fluck M (2014) Hypoxia refines plasticity of mitochondrial respiration to repeated muscle work. Eur J Appl Physiol 114:405–417
Finck BN, Kelly DP (2006) PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Investig 116:615–622
Flueck M (2009) Plasticity of the muscle proteome to exercise at altitude. High Alt Med Biol 10:183–193
Fukuda R, Zhang H, Kim J, Shimoda L, Dang CV, Semenza GL (2007) HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129:111–122
Galbes O, Goret L, Caillaud C, Mercier J, Obert P, Candau R, Py G (2008) Combined effects of hypoxia and endurance training on lipid metabolism in rat skeletal muscle. Acta Physiol 193:163–173
Gamboa JL, Andrade FH (2012) Muscle endurance and mitochondrial function after chronic normobaric hypoxia: contrast of respiratory and limb muscles. Pflugers Arch Eur J Physiol 463:327–338
Granata C, Oliveira RS, Little JP, Renner K, Bishop DJ (2016) Training intensity modulate changes in PGC-1a and p53 protein content and mitochondrial respiration, but not markers of mitochondrial content in human skeletal muscle. FASEB J 30:959–970
Green HJ, Sutton JR, Wolfel EE, Reeves JT, Butterfield GE, Brooks GA (1992) Altitude acclimatization and energy metabolic adaptations in skeletal muscle during exercise. J Appl Physiol 73:2701–2708
Green H, Roy B, Grant S, Burnett M, Tupling R, Otto C, Pipe A, McKenzie D (2000) Downregulation in muscle Na(+)-K(+)-ATPase following a 21-day expedition to 6,194 m. J Appl Physiol 88:634–640
Heather LC, Cole MA, Tan JJ, Ambrose LJA, Pope S, Abd-Jamil AH et al (2012) Metabolic adaptation to chronic hypoxia in cardiac mitochondria. Basic Res Cardiol 107:268–279
Horscroft JA, Burgess SL, Hu Y, Murray AJ (2015) Altered oxygen utilisation in rat left ventricle and soleus after 14 days, but not 2 days, of environmental hypoxia. PLoS One 10(9):e0138564
Jacobs RA, Siebenmann C, Hug M, Toigo M, Mainild A, Lundby C (2012) Twenty-eight days at 3454-m altitude diminishes respiratory capacity but enhances efficiency in human skeletal muscle mitochondria. FASEB J 26:5192–5200
Jacobs RA, Boushel R, Wright-Paradis C, Calbet JA, Robach P, Gnaiger E, Lundby C (2013) Mitochondrial function in human skeletal muscle following high-altitude exposure. Exp Physiol 98:245–255
Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N et al (2012) Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590:3349–3360
Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP (2000) Peroxisome proliferator-activated receptor γ coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Investig 106:847–856
Leverve X (1988) Metabolic and nutritional consequences of chronic hypoxia. Clin Nutr 17:241–251
Mai S, Muster B, Bereiter-Hahn J, Jendrach M (2012) Autophagy proteins LC3B, ATG5and ATG12 participate in quality control after mitochondrial damage and influence lifespan. Autophagy 8:47–62
Meirhaeghe A, Crowley V, Lenaghan C, Lelliot C, Green K, Stewart A, Hart K, Skinner S, Sethi JK, Yeo G, Brand MD, Cortright RN, O’Rahilly S, Montague C, Vidal-Puig AJ (2003) Characterization of the human, mouse and rat PGC1 beta (peroxisome-proliferator-activated receptor-gamma co-activator 1 beta) gene in vitro and in vivo. Biochem J 373:155–165
Nouette-Gaulain K, Malgat M, Rocher C, Savineau JP, Marthan R, Mazat JP, Sztark F (2005) Time course of differential mitochondrial energy metabolism adaptation to chronic hypoxia in right and left ventricles. Cardiovasc Res 66:132–140
Novel-Chaté V, Mateo P, Saks VA, Hoerter JA, Rossi A (1998) Chronic exposure of rats to hypoxic environment alters the mechanism of energy transfer in myocardium. J Mol Cell Cardiol 30:1295–1303
Panariti A, Miserocchi G, Rivolta I (2013) mRNA expression profile of selected oxygen sensing genes in the lung during recovery from chronic hypoxia. Biol Res 46:169–176
Rivolta I, Lucchini V, Rocchetti M, Kolar F, Palazzo F, Zaza A, Miserocchi G (2011) Interstitial pressure and lung oedema in chronic hypoxia. Eur Respir J 37:943–949
Rowe GC, Jiang A, Arany Z (2010) PGC-1 coactivators in cardiac development and disease. Circ Res 107:825–838
Rowe GC, Patten IS, Zsengeller ZK, El-Khoury R, Okutsu M, Bampoh S et al (2013) Disconnecting mitochondrial content from respiratory chain capacity in PGC-1-deficient skeletal muscle. Cell Rep 3:1449–1456
Rumsey WL, Abbott B, Bertelsen D, Mallamaci M, Hagan K, Nelson D, Erecinska M (1999) Adaptation to hypoxia alters energy metabolism in rat heart. Am J of Physiol 276:H71–H80
Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T et al (1998) Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem 184:81–100
Slater EC (1953) Mechanism of phosphorylation in the respiratory chain. Nature 172:975–978
Slot IGM, Schols AMWJ., De Theije CC, Snepvangers FJM, Gosker HR (2016) Alterations in skeletal muscle oxidative phenotype in mice exposed to 3 weeks of normobaric hypoxia. J Cell Physiol 231:377–392
St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM (2003) Bioenergetic analysis of peroxisome proliferator-activated receptor y coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J Biol Chem 278:26597–26603
Sutter CH, Laughner E, Semenza GL (2009) Hypoxia-inducible factor 1alpha protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. Proc Natl Acad Sci USA 97:4748–4753
van Ekeren GJ, Sengers RC, Stadhouders AM (1992) Changes in volume densities and distribution of mitochondria in rat skeletal muscle after chronic hypoxia. Int J Exp Pathol 73:51–60
Wigston DJ, English AW (1992) Fiber-type proportions in mammalian soleus muscle during postnatal development. J Neurobiol 23:61–70
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanism controlling mitochondrial biogenesis and respiration through the termogenic coactivator PGC-1. Cell 98:115–124
Zungu M, Alcolea MP, Garcia-Palmer FJ, Young ME, Essop MF (2007) Genomic modulation of mitochondrial respiratory genes in the hypertrophied heart reflects adaptive changes in mitochondrial and contractile function. Am J Physiol Heart Circ Physiol 293:H2819–H2825
Zungu M, Young ME, Stanley WC, Essop MF (2008) Expression of mitochondrial regulatory genes parallels respiratory capacity and contractile function in a rat model of hypoxia-induced right ventricular hypertrophy. Mol Cell Biochem 318:175–181
Acknowledgements
The authors would like to acknowledge and thank Prof Antonio Zaza, for providing the setup for the study and Riccardo Rizzetto, who cared for the rats. We thank also Dr Simone Porcelli for helping with the mitochondrial respiration analysis.
Funding
This study was funded by Fondo di Ateneo per la Ricerca (FAR)—University of Milano-Bicocca.
Author information
Authors and Affiliations
Contributions
AF, GM, IR and DJB: conception and design; AF, AP, IR and DJB: collection and assembly of data; MR: provision of study materials; GM: funding support; AF, AP, MR, IR, GBC and DJB: data analysis; AF, AP, GM, IR and DJB: data interpretation; AF, AP, IR and DJB: manuscript writing; AF, AP, GM, MR, IR, GBC and DJB: manuscript approval.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Guido Ferretti.
Rights and permissions
About this article
Cite this article
Ferri, A., Panariti, A., Miserocchi, G. et al. Tissue specificity of mitochondrial adaptations in rats after 4 weeks of normobaric hypoxia. Eur J Appl Physiol 118, 1641–1652 (2018). https://doi.org/10.1007/s00421-018-3897-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-018-3897-9