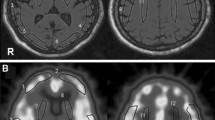
Abstract
White matter lesions in cerebral small vessel disease are related to ischemic injury and increase the risk of stroke and cognitive decline. Pathological changes due to cerebral small vessel disease are increasingly recognized outside of discrete lesions, but the metabolic alterations in nonlesional tissue has not been described. Aerobic glycolysis is critical to white matter myelin homeostasis and repair. In this study, we examined cerebral metabolism of glucose and oxygen as well as blood flow in individuals with and without cerebral small vessel disease using multitracer positron emission tomography. We show that glycolysis is relatively elevated in nonlesional white matter in individuals with small vessel disease relative to healthy, age-matched controls. On the other hand, in young healthy individuals, glycolysis is relatively low in areas of white matter susceptible to lesion formation. These results suggest that increased white matter glycolysis is a marker of pathology associated with small vessel disease.




Similar content being viewed by others
Data availability
Processed data required to generate the figures and tables are included as supplemental information and at https://github.com/matthewbrier/AGinSVD. The AMBR dataset is in the process of being made available for sharing to researchers with an appropriate Data Use Agreement, as required due to Protected Health Information, limitations in the initial consent process and regulations at the Knight Alzheimer Disease Research Center.
Code availability
Image processing toolboxes used in this study are freely available. Specific code to generate the figures and statistical tests are available at https://github.com/matthewbrier/AGinSVD.
References
Prins, N. D. & Scheltens, P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat. Rev. Neurol. 11, 157–165 (2015).
Vernooij, M. W. et al. Incidental findings on brain MRI in the general population. N. Engl. J. Med. 357, 1821–1828 (2007).
Kapasi, A., DeCarli, C. & Schneider, J. A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134, 171–186 (2017).
Debette, S., Schilling, S., Duperron, M. G., Larsson, S. C. & Markus, H. S. Clinical significance of magnetic resonance imaging markers of vascular brain injury: A systematic review and meta-analysis. JAMA Neurol. 76, 81–94 (2019).
Shimony, J. S. et al. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol. Psychiatry 66, 245–252 (2009).
Skrobot, O. A. et al. Vascular cognitive impairment neuropathology guidelines (VCING): The contribution of cerebrovascular pathology to cognitive impairment. Brain 139, 2957–2969 (2016).
Vinters, H. V. et al. Review: Vascular dementia: Clinicopathologic and genetic considerations. Neuropathol. Appl. Neurobiol. 44, 247–266 (2018).
Maillard, P. et al. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke 45, 1721–1726 (2014).
Maillard, P. et al. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. Am. J. Neuroradiol. 34, 54–61 (2013).
Promjunyakul, N. et al. Characterizing the white matter hyperintensity penumbra with cerebral blood flow measures. Neuroimage Clin. 8, 224–229 (2015).
Promjunyakul, N.-O. O. et al. Comparison of cerebral blood flow and structural penumbras in relation to white matter hyperintensities: A multi-modal magnetic resonance imaging study. J. Cereb. Blood Flow. Metab. 36, 1528–1536 (2016).
Reginold, W. et al. Impact of white matter hyperintensities on surrounding white matter tracts. Neuroradiology 60, 933–944 (2018).
Wu, X. et al. Characterizing the penumbras of white matter hyperintensities and their associations with cognitive function in patients with subcortical vascular mild cognitive impairment. Front Neurol. 10, 1–10 (2019).
Maillard, P. et al. White matter hyperintensity penumbra. Stroke 42, 1917–1922 (2011).
Nasrallah, I. M. et al. White matter lesion penumbra shows abnormalities on structural and physiologic MRIs in the coronary artery risk development in young adults cohort. Am. J. Neuroradiol. 40, 1291–1298 (2019).
Fünfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443 (2012).
Li, F. et al. Glial metabolic rewiring promotes axon regeneration and functional recovery in the central nervous system. Cell Metab. 32, 767–785.e7 (2020).
Goyal, M. S. et al. Brain aerobic glycolysis and resilience in Alzheimer disease. Preprint at bioRxiv https://doi.org/10.1101/2022.06.21.497006 (2022).
Scheltens, P. et al. White matter changes on CT and MRI: An overview of visual rating scales. Eur. Neurol. 39, 80–89 (1998).
Vaishnavi, S. N. et al. Regional aerobic glycolysis in the human brain. Proc. Natl Acad. Sci. USA 107, 17757–17762 (2010).
Rowland, D. J., Garbow, J. R., Laforest, R. & Snyder, A. Z. Registration of [18F]FDG microPET and small-animal MRI. Nucl. Med. Biol. 32, 567–572 (2005).
Brier, M. R. et al. Quantitative signal properties from standardized MRIs correlate with multiple sclerosis disability. Ann. Clin. Transl. Neurol. 8, 1096–1109 (2021).
Fischl, B. FreeSurfer. NeuroImage 62, 774–781 (2012).
Sattarivand, M., Kusano, M., Poon, I. & Caldwell, C. Symmetric geometric transfer matrix partial volume correction for PET imaging: Principle, validation and robustness. Phys. Med. Biol. 57, 7101–7116 (2012).
Erlandsson, K., Buvat, I., Pretorius, P. H., Thomas, B. A. & Hutton, B. F. A review of partial volume correction techniques for emission tomography and their applications in neurology, cardiology and oncology. Phys. Med. Biol. 57, R119–R159 (2012).
De Reuck, J. The human periventricular arterial blood supply and the anatomy of cerebral infarctions. Eur. Neurol. 5, 321–334 (1971).
Sundaresan, V. et al. Modelling the distribution of white matter hyperintensities due to ageing on MRI images using Bayesian inference. Neuroimage 185, 434–445 (2019).
Liu, H. et al. Aging of cerebral white matter. Ageing Res Rev. 34, 64–76 (2017).
Goyal, M. S. et al. Loss of brain aerobic glycolysis in normal human aging. Cell Metab. 26, 353–360.e3 (2017).
Morland, C., Henjum, S., Iversen, E. G., Skrede, K. K. & Hassel, B. Evidence for a higher glycolytic than oxidative metabolic activity in white matter of rat brain. Neurochem. Int. 50, 703–709 (2007).
Hyder, F. et al. Uniform distributions of glucose oxidation and oxygen extraction in gray matter of normal human brain: No evidence of regional differences of aerobic glycolysis. J. Cereb. Blood Flow. Metab. 36, 903–916 (2015).
Nave, K. A. Myelination and support of axonal integrity by glia. Nature 468, 244–252 (2010). Preprint at.
Supplie, L. M. et al. Respiration-deficient astrocytes survive as glycolytic cells in vivo. J. Neurosci. 37, 4231–4242 (2017).
Kang, P. et al. Oxygen metabolic stress and white matter injury in patients with cerebral small vessel disease. Stroke 53, 1570–1579 (2022).
Majmundar, A. J., Wong, W. J. & Simon, M. C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 (2010).
Soto-Heredero, G., Gómez de las Heras, M. M., Gabandé-Rodríguez, E., Oller, J. & Mittelbrunn, M. Glycolysis – a key player in the inflammatory response. FEBS J. 287, 3350–3369 (2020).
Zuend, M. et al. Arousal-induced cortical activity triggers lactate release from astrocytes. Nat. Metab. 2020 2:2 2, 179–191 (2020).
Brown, A. M. & Ransom, B. R. Astrocyte glycogen as an emergency fuel under conditions of glucose deprivation or intense neural activity. Metab. Brain Dis. 30, 233–239 (2015).
Nave, K. A. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 11, 275–283 (2010).
Dolui, S. et al. Characterizing a perfusion-based periventricular small vessel region of interest. Neuroimage Clin. 23, 101897 (2019).
de Havenon, A. et al. Blood pressure, glycemic control, and white matter hyperintensity progression in type 2 diabetics. Neurology 92, E1168–E1175 (2019).
Schulkin, J. & Sterling, P. Allostasis: a brain-centered, predictive mode of physiological regulation. Trends Neurosci. 42, 740–752 (2019).
Chimowitz, M. I., Estes, M. L., Furlan, A. J. & Awad, I. A. Further observations on the pathology of subcortical lesions identified on magnetic resonance imaging. Arch. Neurol. 49, 747–752 (1992).
Gouw, A. A. et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J. Neurol. Neurosurg. Psychiatry 82, 126–135 (2011).
Sosa, S. M. & Smith, K. J. Understanding a role for hypoxia in lesion formation and location in the deep and periventricular white matter in small vessel disease and multiple sclerosis. Clin. Sci. 131, 2503–2524 (2017).
Cho, S. H. et al. Appropriate reference region selection of 18F-florbetaben and 18F-flutemetamol beta-amyloid PET expressed in Centiloid. Sci. Rep. 10, 1–10 (2020).
Minoshima, S., Frey, K. A., Foster, N. L. & Kuhl, D. E. Preserved pontine glucose metabolism in alzheimer disease: A reference region for functional brain image (pet) analysis. J. Comput. Assist. Tomogr. 19, 541–547 (1995).
Giralt-Steinhauer, E. et al. Brainstem leukoaraiosis independently predicts poor outcome after ischemic stroke. Eur. J. Neurol. 25, 1086–1092 (2018).
Acknowledgements
We are grateful to our research participants and their families for their altruism. We appreciate VG Lab members for their efforts in participant recruitment and acquiring, processing and assembling the AMBR dataset19. We also acknowledge the Neuroimaging Labs Research Center, Knight Alzheimer’s Disease Research Center, cyclotron and imaging staff for making this research possible. Funding for this research was provided by the National Institutes of Health/National Institute on Aging RF1AG073210 (A.G.V. and M.S.G.), R01AG057536 (A.G.V. and M.S.G.), R01AG053503 (A.G.V. and M.E.R.), P50AG005681 (J.C.M.), P01AG026276 (J.C.M.) and P01AG003991 (J.C.M. and T.L.S.B.). M.R.B. was supported by grants R25NS090978 and KL2TR002346 from NIH. Some of the MRI sequences were obtained from the Massachusetts General Hospital.
Author information
Authors and Affiliations
Contributions
M.R.B. contributed to developing the hypothesis, designed and implemented the analytic strategy, performed the analysis and statistical inferences and drafted and revised the manuscript. T.B. processed imaging data, performed initial analyses, developed image processing tools and revised the manuscript. M.E.R. conceived of the study, assisted in the design and critically revised the manuscript. J.C.M. contributed to data collection, supervised the overarching study and revised the manuscript. T.L.S.B. contributed to data collection and technical image analysis, provided image processing tools and revised the manuscript. A.G.V. designed the study, supervised data collection, curated the data, assisted with the analysis and revised the manuscript. A.Z.S. developed tools for the analysis, contributed to the design of the analytic strategy and revised the manuscript. M.S.G. conceived of the study, hypothesis and analytic approach, as well as drafted and revised the manuscript. M.R.B. and M.S.G. are responsible for the content of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
T.L.S.B. and M.S.G. have received sponsored research grant support from Siemens Healthineers AG. The other authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Audrey (P.) Fan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplemental Tables 1 – 4; Supplemental Figures 1 – 2
Source data
Source Data Fig. 1
Single-subject measurements of metabolic parameters per tissue type.
Source Data Fig. 2
Single-subject measures of metabolic parameters parametric in lesion threshold; single-subject model fits of metabolic parameters in each tissue compartment.
Source Data Fig. 3
Single-subject measurements of each metabolic parameter parametric in d for each subpanel of the figure.
Source Data Fig. 4
Single-subject measurements in the WMv and WMn ROI at different fractional cutoffs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brier, M.R., Blazey, T., Raichle, M.E. et al. Increased white matter glycolysis in humans with cerebral small vessel disease. Nat Aging 2, 991–999 (2022). https://doi.org/10.1038/s43587-022-00303-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-022-00303-y
- Springer Nature America, Inc.