Abstract
Recent proteome and transcriptome profiling of Alzheimer’s disease (AD) brains reveals RNA splicing dysfunction and U1 small nuclear ribonucleoprotein (snRNP) pathology containing U1-70K and its N-terminal 40-KDa fragment (N40K). Here we present a causative role of U1 snRNP dysfunction to neurodegeneration in primary neurons and transgenic mice (N40K-Tg), in which N40K expression exerts a dominant-negative effect to downregulate full-length U1-70K. N40K-Tg recapitulates N40K insolubility, erroneous splicing events, neuronal degeneration and cognitive impairment. Specifically, N40K-Tg shows the reduction of GABAergic synapse components (for example, the GABA receptor subunit of GABRA2) and concomitant postsynaptic hyperexcitability that is rescued by a GABA receptor agonist. Crossing of N40K-Tg and the 5xFAD amyloidosis model indicates that the RNA splicing defect synergizes with the amyloid cascade to remodel the brain transcriptome and proteome, deregulate synaptic proteins and accelerate cognitive decline. Thus, our results support the contribution of U1 snRNP-mediated splicing dysfunction to AD pathogenesis.







Similar content being viewed by others
Data availability
ROSMAP data can be download from this website (https://adknowledgeportal.synapse.org/) and mapped to human transcriptome (hg19). All mouse RNA-seq data were deposited in the GEO database under accession codes GSE115177 (mouse brain) and GSE196873 containing two sub-series IDs, GSE196871 (mouse brain) and GSE196872 (mouse neuronal culture) and mapped to the mouse transcriptome (mm9). The MS proteomics data were deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifiers PXD031587 (human aggregated proteome), PXD031581 (mouse aggregated proteome), PXD031546 (mouse neuronal culture) and PXD023395 and PXD031545 (mouse brain). Both hg19 and mm9 can be download from UCSC website (https://genome.ucsc.edu/index.html). Other data underlying the paper are provided as source data files or are available from the corresponding author upon reasonable request.
Code availability
The source codes are available at https://github.com/PengLabStJude/N40K_model.
References
Raj, B. & Blencowe, B. J. Alternative splicing in the mammalian nervous system: recent insights into mechanisms and functional roles. Neuron 87, 14–27 (2015).
Cooper, T. A., Wan, L. & Dreyfuss, G. RNA and disease. Cell 136, 777–793 (2009).
Scotti, M. M. & Swanson, M. S. RNA mis-splicing in disease. Nat. Rev. Genet. 17, 19–32 (2016).
Mathieu, C., Pappu, R. V. & Taylor, J. P. Beyond aggregation: pathological phase transitions in neurodegenerative disease. Science 370, 56–60 (2020).
Lefebvre, S. et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80, 155–165 (1995).
Mackenzie, I. R., Rademakers, R. & Neumann, M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 9, 995–1007 (2010).
Nguyen, H. P., Van Broeckhoven, C. & van der Zee, J. ALS genes in the genomic era and their implications for FTD. Trends Genet. 34, 404–423 (2018).
Jutzi, D. et al. Aberrant interaction of FUS with the U1 snRNA provides a molecular mechanism of FUS induced amyotrophic lateral sclerosis. Nat. Commun. 11, 6341 (2020).
Yu, Y. et al. U1 snRNP is mislocalized in ALS patient fibroblasts bearing NLS mutations in FUS and is required for motor neuron outgrowth in zebrafish. Nucleic Acids Res. 43, 3208–3218 (2015).
Sun, S. et al. ALS-causative mutations in FUS/TLS confer gain and loss of function by altered association with SMN and U1-snRNP. Nat. Commun. 6, 6171 (2015).
Jia, Y., Mu, J. C. & Ackerman, S. L. Mutation of a U2 snRNA gene causes global disruption of alternative splicing and neurodegeneration. Cell 148, 296–308 (2012).
Josephs, K. A. et al. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol. 127, 441–450 (2014).
Hyman, B. T. et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 8, 1–13 (2012).
DeTure, M. A. & Dickson, D. W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. https://doi.org/10.1186/s13024-019-0333-5 (2019).
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991).
Selkoe, D. J. Alzheimer’s disease is a synaptic failure. Science 298, 789–791 (2002).
Aebersold, R. & Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 537, 347–355 (2016).
Bai, B. et al. Proteomic landscape of Alzheimer’s disease: novel insights into pathogenesis and biomarker discovery. Mol. Neurodegener. 16, 55 (2021).
Bai, B. et al. U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 110, 16562–16567 (2013).
Johnson, E. C. B. et al. Deep proteomic network analysis of Alzheimer’s disease brain reveals alterations in RNA binding proteins and RNA splicing associated with disease. Mol. Neurodegener. 13, 52 (2018).
Bai, B. et al. Deep multilayer brain proteomics identifies molecular networks in Alzheimer’s disease progression. Neuron 105, 975–991 (2020).
Yu, K. et al. Global profiling of lysine accessibility to evaluate protein structure changes in Alzheimer’s disease. J. Am. Soc. Mass. Spectrom. 32, 936–945 (2021).
Raj, T. et al. Integrative transcriptome analyses of the aging brain implicate altered splicing in Alzheimer’s disease susceptibility. Nat. Genet. 50, 1584–1592 (2018).
Hales, C. M. et al. Changes in the detergent-insoluble brain proteome linked to amyloid and tau in Alzheimer’s disease progression. Proteomics 16, 3042–3053 (2016).
Lutz, B. M. & Peng, J. Deep profiling of the aggregated proteome in Alzheimer’s disease: from pathology to disease mechanisms. Proteomes 6, 46 (2018).
Pomeranz Krummel, D. A., Oubridge, C., Leung, A. K., Li, J. & Nagai, K. Crystal structure of human spliceosomal U1 snRNP at 5.5 A resolution. Nature 458, 475–480 (2009).
Hales, C. M. et al. Aggregates of small nuclear ribonucleic acids (snRNAs) in Alzheimer’s disease. Brain Pathol. 24, 344–351 (2014).
Hales, C. M. et al. U1 small nuclear ribonucleoproteins (snRNPs) aggregate in Alzheimer’s disease due to autosomal dominant genetic mutations and trisomy 21. Mol. Neurodegener. 9, 15 (2014).
Bai, B. et al. Integrated approaches for analyzing U1-70K cleavage in Alzheimer’s disease. J. Proteome Res. 13, 4526–4534 (2014).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Bai, B. et al. Deep profiling of proteome and phosphoproteome by isobaric labeling, extensive liquid chromatography, and mass spectrometry. Methods Enzymol. 585, 377–395 (2017).
Wang, Z. et al. 27-plex tandem mass tag mass spectrometry for profiling brain proteome in Alzheimer’s disease. Anal. Chem. 92, 7162–7170 (2020).
Diner, I. et al. Aggregation properties of the small nuclear ribonucleoprotein U1-70K in Alzheimer disease. J. Biol. Chem. 289, 35296–35313 (2014).
Bishof, I. et al. RNA-binding proteins with basic-acidic dipeptide (BAD) domains self-assemble and aggregate in Alzheimer’s disease. J. Biol. Chem. 293, 11047–11066 (2018).
Kondo, Y., Oubridge, C., van Roon, A. M. & Nagai, K. Crystal structure of human U1 snRNP, a small nuclear ribonucleoprotein particle, reveals the mechanism of 5′ splice site recognition. eLife https://doi.org/10.7554/elife.04986 (2015).
Sigel, E. & Steinmann, M. E. Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 287, 40224–40231 (2012).
Yuan, J., Amin, P. & Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 20, 19–33 (2019).
Guo, T. et al. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 15, 40 (2020).
Dittgen, T. et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl Acad. Sci. USA 101, 18206–18211 (2004).
Uezu, A. et al. Identification of an elaborate complex mediating postsynaptic inhibition. Science 353, 1123–1129 (2016).
Oleskevich, S., Leck, K. J., Matthaei, K. & Hendry, I. A. Enhanced serotonin response in the hippocampus of Galphaz protein knock-out mice. Neuroreport 16, 921–925 (2005).
Schwindinger, W. F. et al. Loss of G protein γ 7 alters behavior and reduces striatal α(olf) level and cAMP production. J. Biol. Chem. 278, 6575–6579 (2003).
Simons, C. et al. Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple–Baraitser syndrome and epilepsy. Nat. Genet. 47, 73–77 (2015).
Floudas, C. S., Um, N., Kamboh, M. I., Barmada, M. M. & Visweswaran, S. Identifying genetic interactions associated with late-onset Alzheimer’s disease. BioData Min. 7, 35 (2014).
Limon, A., Reyes-Ruiz, J. M. & Miledi, R. Loss of functional GABA(A) receptors in the Alzheimer diseased brain. Proc. Natl Acad. Sci. USA 109, 10071–10076 (2012).
Hyman, B. T., Van Hoesen, G. W., Kromer, L. J. & Damasio, A. R. Perforant pathway changes and the memory impairment of Alzheimer’s disease. Ann. Neurol. 20, 472–481 (1986).
Scheff, S. W. & Price, D. A. Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J. Alzheimers Dis. 9, 101–115 (2006).
Tyagarajan, S. K. & Fritschy, J. M. Gephyrin: a master regulator of neuronal function? Nat. Rev. Neurosci. 15, 141–156 (2014).
Chacon, P. J. et al. Cerebellin 4, a synaptic protein, enhances inhibitory activity and resistance of neurons to amyloid-β toxicity. Neurobiol. Aging 36, 1057–1071 (2015).
Upadhyay, A. et al. Neurocalcin δ knockout impairs adult neurogenesis whereas half reduction is not pathological. Front. Mol. Neurosci. 12, 19 (2019).
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007).
Wan, Y. W. et al. Meta-analysis of the Alzheimer’s disease human brain transcriptome and functional dissection in mouse models. Cell Rep. 32, 107908 (2020).
Johnson, E. C. B. et al. Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. https://doi.org/10.1038/s41591-020-0815-6 (2020).
Wang, T. et al. Identification and characterization of essential genes in the human genome. Science 350, 1096–1101 (2015).
Salz, H. K. et al. The Drosophila U1-70K protein is required for viability, but its arginine-rich domain is dispensable. Genetics 168, 2059–2065 (2004).
Xue, S. et al. Low-complexity domain of U1-70K modulates phase separation and aggregation through distinctive basic-acidic motifs. Sci. Adv. 5, eaax5349 (2019).
Winklhofer, K. F., Tatzelt, J. & Haass, C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J. 27, 336–349 (2008).
Scheper, W. & Hoozemans, J. J. The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathol. 130, 315–331 (2015).
Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 (2012).
Ranasinghe, K. G. et al. Neurophysiological signatures in Alzheimer’s disease are distinctly associated with tau, amyloid-β accumulation, and cognitive decline. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaz4069 (2020).
Grubman, A. et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22, 2087–2097 (2019).
Palop, J. J. & Mucke, L. Amyloid-β-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat. Neurosci. 13, 812–818 (2010).
Hsieh, Y. C. et al. Tau-mediated disruption of the spliceosome triggers cryptic RNA splicing and neurodegeneration in Alzheimer’s disease. Cell. Rep. 29, 301–316 (2019).
Apicco, D. J. et al. Dysregulation of RNA splicing in tauopathies. Cell. Rep. 29, 4377–4388 (2019).
Lester, E. et al. Tau aggregates are RNA-protein assemblies that mislocalize multiple nuclear speckle components. Neuron 109, 1675–1691 (2021).
Giasson, B. I. et al. Initiation and synergistic fibrillization of tau and α-synuclein. Science 300, 636–640 (2003).
Nelson, P. T. et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 142, 1503–1527 (2019).
Beach, T. G. et al. Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 35, 354–389 (2015).
Kosse, C. & Burdakov, D. Natural hypothalamic circuit dynamics underlying object memorization. Nat. Commun. 10, 2505 (2019).
Achilly, N. P., Wang, W. & Zoghbi, H. Y. Presymptomatic training mitigates functional deficits in a mouse model of Rett syndrome. Nature 592, 596–600 (2021).
Bai, B. et al. Deep multilayer brain proteomics identifies molecular networks in Alzheimer’s disease progression. Neuron 105, 975–991 (2020).
Lois, C., Hong, E. J., Pease, S., Brown, E. J. & Baltimore, D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295, 868–872 (2002).
Xu, P., Duong, D. M. & Peng, J. Systematical optimization of reverse-phase chromatography for shotgun proteomics. J. Proteome Res. 8, 3944–3950 (2009).
Wang, H. et al. Systematic optimization of long gradient chromatography mass spectrometry for deep analysis of brain proteome. J. Proteome Res. 14, 829–838 (2015).
Wang, X. et al. JUMP: a tag-based database search tool for peptide identification with high sensitivity and accuracy. Mol. Cell Proteomics 13, 3663–3673 (2014).
Peng, J., Elias, J. E., Thoreen, C. C., Licklider, L. J. & Gygi, S. P. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J. Proteome Res. 2, 43–50 (2003).
Elias, J. E. & Gygi, S. P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 (2007).
Niu, M. et al. Extensive peptide fractionation and y1 ion-based interference detection method for enabling accurate quantification by isobaric labeling and mass spectrometry. Anal. Chem. 89, 2956–2963 (2017).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Mertens, B. J. A. Transformation, Normalization, and Batch Effect in the Analysis of Mass Spectrometry Data for Omics Studies. Statistical Analysis of Proteomics, Metabolomics, and Lipidomics Data Using Mass Spectrometry 1–21 (2017).
Rusch, M. et al. Clinical cancer genomic profiling by three-platform sequencing of whole genome, whole exome and transcriptome. Nat. Commun. 9, 3962 (2018).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014).
Vanderwall, D. et al. JUMPn: a streamlined application for protein co-expression clustering and network analysis in proteomics. J. Vis. Exp. https://doi.org/10.3791/62796 (2021).
Tan, H. et al. Integrative proteomics and phosphoproteomics profiling reveals dynamic signaling networks and bioenergetics pathways underlying T cell activation. Immunity 46, 488–503 (2017).
Szklarczyk, D. et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015).
Huttlin, E. L. et al. The BioPlex network: a systematic exploration of the human interactome. Cell 162, 425–440 (2015).
Li, T. et al. A scored human protein-protein interaction network to catalyze genomic interpretation. Nat. Methods 14, 61–64 (2017).
Barabasi, A. L. & Oltvai, Z. N. Network biology: understanding the cell’s functional organization. Nat. Rev. Genet. 5, 101–113 (2004).
Ravasz, E., Somera, A. L., Mongru, D. A., Oltvai, Z. N. & Barabasi, A. L. Hierarchical organization of modularity in metabolic networks. Science 297, 1551–1555 (2002).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 9, 559 (2008).
Wang, F. et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 14, 22–29 (2012).
West, M. J., Slomianka, L. & Gundersen, H. J. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231, 482–497 (1991).
Chapman, P. F. et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 2, 271–276 (1999).
Uslaner, J. M. et al. The muscarinic M1 receptor positive allosteric modulator PQCA improves cognitive measures in rat, cynomolgus macaque, and rhesus macaque. Psychopharmacology 225, 21–30 (2013).
Heneka, M. T. et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013).
Prusky, G. T., Alam, N. M., Beekman, S. & Douglas, R. M. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest. Ophthalmol. Vis. Sci. 45, 4611–4616 (2004).
Bayazitov, I. T., Richardson, R. J., Fricke, R. G. & Zakharenko, S. S. Slow presynaptic and fast postsynaptic components of compound long-term potentiation. J. Neurosci. 27, 11510–11521 (2007).
Acknowledgements
We thank all other laboratory and center members for discussion and technical support. We thank St Jude Shared Resources and Core Facilities, including the Animal Research Center, Transgenic/Gene Knockout, In Vivo Imaging and Therapeutics, Veterinary Pathology, Cytogenetics, Genome Sequencing, Proteomics and Metabolomics, Applied Bioinformatics and Cell and Tissue Imaging. We also thank J. Jankowsky for providing plasmids, I. Bayazitov for electrophysiology, F. Zheng for the discussion of behavior studies, A. Hemphill for western blots of replicates during revision and I. Chen for helpful discussion and guidance. This work was partially supported by National Institutes of Health grants R01AG047928 (J.P.), R01AG053987 (J.P.), RF1AG068581 (J.P.), RF1AG064909 (G.Y. and J.P.), U54NS110435 (J.P.), U19AG069701 (J.P.), R01MH095810 (S.S.Z.) and American Lebanese Syrian Associated Charities. The Banner Sun Health Research Institute Brain and Body Donation Program was supported by National Institutes of Health grants U24NS072026, P30AG072980, P30AG19610, the Arizona Department of Health Services, the Arizona Biomedical Research Commission and the Michael J. Fox Foundation for Parkinson’s Research.
Author information
Authors and Affiliations
Contributions
J.P., P.-C.C., X.H. and G.Y. conceived the project. P.-C.C. generated the N40K-Tg mouse model. P.-C.C., X.H., H.S., M.N., Y.J., B.J.W.T., D.E., L.B., B.B., J.M., Z.W., D.L., J.L., H.-M.L., A.M., L.R., M.H.P., L.R.E., E.S. and P.V. performed the experiments. G.E.S., T.G.B. and D.A.B. characterized and provided human brain samples. X.H., P.-C.C., T.I.S., Y.F., Y.L., J.-H.C., X.W., S.P., Z.-F.Y., Y.H., S.W., M.A.D., R.J.S., T.M., T.C., G.W., S.S.Z., G.Y. and J.P. analyzed the data. X. H., P.-C.C. and J.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Benjamin Logsdon, Benjamin Wolozin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Deep TMT profiling of detergent-insoluble proteome in AD and quantitative analysis of western blotting.
a, Proteomic profiling of detergent-insoluble proteome of 10 control and 10 AD cases by TMT-LC/LC-MS/MS. A total of 8,917 proteins were identified, out of which 365 proteins were found to increase in AD detergent-insoluble proteome. b, Principal component analysis of the insoluble proteome in control and AD cases. c, Heatmap of selected top proteins including U1 snRNP components enriched in AD. d, Pathway enrichment analysis of the proteins enriched in the AD insoluble proteome (Fisher’s exact test and BH procedure to generate FDR values). e, Enriched protein-protein interaction module of spliceosome. f, Quantified data of western blotting (4 replicates) in Fig. 1d (Student’s t-test, two-tailed). g, Quantified data of western blotting (triplicates) in Fig. 1e to show that the N40K expression leads to the depletion of endogenous U1-70K in neurons (Student’s t-test, two-tailed). Data are shown as mean ± SEM. Full statistical information is in Source Data Statistics
Extended Data Fig. 2 Generation of multiple N40K-Tg lines with N40K expression and U1-70K downregulation.
a, The strategy for producing N40K-Tg lines by the injection of N40-expressing lentivirus. b, Chromosomal localization of Tg determined by fluorescence in situ hybridization (FISH) analysis. Left: Chr18 (green), N40K (red) in Tg396 line; Right: Chr10 (green), N40K (red) in Tg318 line. c, Western blotting of U1-70K and N40K in Tg396 and Tg318 Tg lines. N40K expression led to similar depletion of U1-70K protein in the hippocampus of both lines. d, Quantitation of relative N40K levels in cortex and hippocampus of Tg396 lines by western blotting. Titrated Tg proteins produce a linear response curve (R2 = 0.98). According to the curve equation, N40K is at a ~2-fold level of native U1-70K as in WT mice (Student’s t-test, two-tailed). Data are shown as mean ± SEM. In b, c, d, the assay was repeated three times. Full statistical information is in Source Data Statistics.
Extended Data Fig. 3 Proteomics analysis of insoluble fraction in WT and N40K-Tg mice.
a, Proteomic profiling of detergent-insoluble proteome of WT and N40K-Tg mice (12-month-old, WT n = 3, Tg n = 3). b, Principal component analysis of the insoluble proteome of WT and N40K. c, Heatmap of differentially expressed proteins in N40K-Tg insoluble fraction. d, Pathway enrichment analysis of differentially expressed proteins in N40K-Tg insoluble fraction (Fisher’s exact test) followed by FDR analysis by the BH procedure. e, Enriched PPI module of spliceosomal snRNP complex (Fisher’s exact test and the BH procedure, FDR < 0.05). Each dot represents a protein, whereas the interactions are indicated by connected lines.
Extended Data Fig. 4 N40K-Tg mice exhibit brain weight loss and brain volume reduction, but with normal locomotive activities.
a-c, N40K-Tg mice showed the reduction of brain weight and brain volume, whereas the body weight had no change at different ages (3-month-old: WT n = 14, Tg n = 10; 12-month-old: WT n = 14, Tg n = 11, Student’s t-test, two- tailed). d, Volume change of Tg318 mouse in cortex and hippocampus (Hipp) measured by MRI at different ages (3-month-old: WT n = 15, Tg n = 10; 12-month-old: WT n = 15, Tg n = 10, two-way ANOVA followed by Sidak’s multiple comparison test). e, Morris water maze task for Tg318 mice. Compared to WT control, Tg318 mice showed significant difference at day 3-6 (two-way ANOVA followed by Sidak’s multiple comparison test). f, Speed in the probe trial of Morris water maze at day 6 showed no significant difference between WT and Tg mice in Fig. 4g (12-month-old: WT n = 9, Tg n = 9, Student’s t-test, two-tailed). Data are shown as mean ± SEM, ns (not significant). Full statistical information is in Source Data Statistics.
Extended Data Fig. 5 Validation of splicing deficient transcripts of synaptic pathway in N40K-Tg mice.
a-b, Quantitation of intron accumulation at the selected region of Gabra2, Gng7, Kcnh1 and Camk1d. IGV software was used to display RNA read density with same scale range in each gene from RNA-seq (ex: exon; in: intron). Red boxes show selected regions for quantification. Intron reads % was analyzed by RNA-seq ((intron-exon junctions + introns)/(intron-exon junctions + introns + exons + exon-exon junctions)). Intron retention % was analyzed by RT-PCR ((intron-containing PCR band intensity)/(intron-containing PCR band intensity + exon-exon PCR band intensity)). Gabra2: intron 6 vs exon 6 and 7; Gng7: intron 2 vs exon 2 and 3, Kcnh1: intron 9 vs exon 9 and 10 and Camk1d: intron 5 vs exon 5 and 6. 12-month-old: WT n = 3, Tg n = 3. Scale bar, 10 kb. Data are shown as mean ± SEM. Statistical significance was analyzed by Student’s t-test, two-tailed. Data are shown as mean ± SEM. Full statistical information is in Source Data Statistics.
Extended Data Fig. 6 Profiling analysis of dTg and tau mice.
a, Representative co-immunofluorescence staining of 5xFAD and dTg brain slides with Thioflavin S for plaques and the U1-70K C-terminal antibody for U1-70K depletion. Scale bar, 100 µm. The immunostaining was repeated from three animals. b, PCA for RNA-seq and proteomics studies. c, Distribution of splicing deficiency scores of mapped transcripts. Statistical comparisons between different genotypes are shown (Kolmogorov–Smirnov test). d, Relative Aβ level in 5xFAD and dTg by the proteomics analysis (mean ± SEM, Student’s t-test, two-tailed, ns: not significant). e, Cell type enriched DE proteins in the dTg mice. The 979 DE proteins were overlapped with the cell type expression data from RNA-seq analysis99. f, Swimming speed of WT, N40K-Tg, 5xFAD, dTg in the Morris water maze experiment (mean ± SEM, one-way ANOVA, ns: not significant). g, RNA-seq analysis of WT and Tau (P301S) mice. h, The percentage of mapped intron reads in all transcripts from the cortices of WT (n = 5) and Tau P301S (n = 6) mice (mean ± SEM, Student’s t-test, two-tailed, ns: not significant). i, Distribution of splicing deficiency scores of mapped transcripts (Kolmogorov–Smirnov test). Full statistical information is in Source Data Statistics.
Extended Data Fig. 7 Proteomic comparison of the three mouse models and AD.
a, The workflow to examine the consistent changes in the three mouse models and human AD cases by overlapping differentially expressed proteins. b, DE protein numbers in the mouse models, human cases and the overlapped portions. c, Enriched pathways of the human-overlapped DE proteins in three genotypes (selected from supplementary table 19, Fisher’s exact test and the BH procedure to derive FDR, FDR cutoff of 0.05). d, Enriched protein-protein interaction modules (selected from supplementary table 20, Fisher’s exact test and the BH procedure to derive FDR). FDR cutoff is 0.05 except the Aβ binding module, which is selected due to biological significance.
Extended Data Fig. 8
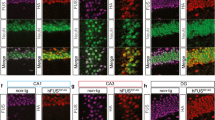
Analysis of the role of TDP-43 in human cases and the mouse models. a, The weak correlation between the percentage of intron reads and the stages of TDP-43 pathology. Pearson correlation coefficient (r) is shown. b, The percentage of mapped intron reads in all transcripts from human cases of different TDP-43 stages (mean ± SEM, Student’s t-test, two-tailed, ns: not significant). As the sample size was small, we merged stages 0-1 and stages 2–3 in the analysis. c, Distribution of splicing deficiency scores of mapped transcripts (Kolmogorov–Smirnov test). d, Staining of plaques, Tau, TDP-43 and nuclei in a human AD brain sample (positive controls) and in the mouse models (cortex, ~12-month-old). Phosphorylated Tau and TDP-43 antibodies were used. The immunostaining was repeated from three human cases or animals. Full statistical information is in Source Data Statistics.
Supplementary information
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Fig. 1
Uncropped western blots and gels.
Source Data Fig. 2
Uncropped western blots and gels.
Source Data Fig. 3
Uncropped western blots and gels.
Source Data Fig. 5
Uncropped western blots and gels.
Source Data Fig. 6
Uncropped western blots and gels.
Source Data Fig. 7
Uncropped western blots and gels.
Source Data Fig. 5
Uncropped western blots and gels.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, PC., Han, X., Shaw, T.I. et al. Alzheimer’s disease-associated U1 snRNP splicing dysfunction causes neuronal hyperexcitability and cognitive impairment. Nat Aging 2, 923–940 (2022). https://doi.org/10.1038/s43587-022-00290-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-022-00290-0
- Springer Nature America, Inc.