Abstract
We report the results of 24 women, 50% (N = 12) with hormone receptor-positive breast cancer and 50% (N = 12) with advanced triple-negative breast cancer, treated with entinostat + nivolumab + ipilimumab from the dose escalation (N = 6) and expansion cohort (N = 18) of ETCTN-9844 (NCT02453620). The primary endpoint was safety. Secondary endpoints were overall response rate, clinical benefit rate, progression-free survival and change in tumor CD8:FoxP3 ratio. There were no dose-limiting toxicities. Among evaluable participants (N = 20), the overall response rate was 25% (N = 5), with 40% (N = 4) in triple-negative breast cancer and 10% (N = 1) in hormone receptor-positive breast cancer. The clinical benefit rate was 40% (N = 8), and progression-free survival at 6 months was 50%. Exploratory analyses revealed that changes in myeloid cells may contribute to responses; however, no correlation was noted between changes in CD8:FoxP3 ratio, PD-L1 status and tumor mutational burden and response. These findings support further investigation of this treatment in a phase II trial.






Similar content being viewed by others
Data availability
All of the datasets generated during the current study that can be shared are available as Supplementary Tables. Whole-exome sequencing data are not publicly available due to lack of consent for data sharing. Source data are provided with this paper and are also available at https://github.com/FertigLab/J15221_trial_paper/tree/main/input_tables.
Code availability
All code used for analyses is available on GitHub at https://github.com/FertigLab/J15221_trial_paper.
References
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Stanton, S. E., Adams, S. & Disis, M. L. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2, 1354–1360 (2016).
Vikas, P., Borcherding, N. & Zhang, W. The clinical promise of immunotherapy in triple-negative breast cancer. Cancer Manag. Res. 10, 6823–6833 (2018).
Agarwala, S. S. et al. Efficacy and safety of entinostat (ENT) and pembrolizumab (PEMBRO) in patients with melanoma progressing on or after a PD-1/L1 blocking antibody. J. Clin. Oncol. 36, 9530 (2018).
Emens, L. A. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of breast cancer. J. Immunother. Cancer 9, e002597 (2021).
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 26, 259–271 (2015).
Schmid, P. et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
Cortes, J. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396, 1817–1828 (2020).
O’Shaughnessy, J. et al. Results of ENCORE 602 (TRIO025), a phase II, randomized, placebo-controlled, double-blinded, multicenter study of atezolizumab with or without entinostat in patients with advanced triple-negative breast cancer (aTNBC). J. Clin. Oncol. 38, 1014 (2020).
Terranova-Barberio, M. et al. Exhausted T cell signature predicts immunotherapy response in ER-positive breast cancer. Nat. Commun. 11, 3584 (2020).
Kim, K. et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc. Natl Acad. Sci. USA 111, 11774–11779 (2014).
Shen, L. et al. Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS ONE 7, e30815 (2012).
Orillion, A. et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin. Cancer Res. 23, 5187–5201 (2017).
Torres, E. T. R. & Emens, L. A. Emerging combination immunotherapy strategies for breast cancer: dual immune checkpoint modulation, antibody–drug conjugates and bispecific antibodies. Breast Cancer Res. Treat. 191, 291–302 (2021).
Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 38, 255 (2019).
Santa-Maria, C. A. et al. A pilot study of durvalumab and tremelimumab and immunogenomic dynamics in metastatic breast cancer. Oncotarget 9, 18985–18996 (2018).
Christmas, B. J. et al. Entinostat converts immune-resistant breast and pancreatic cancers into checkpoint-responsive tumors by reprogramming tumor-infiltrating MDSCs. Cancer Immunol. Res. 6, 1561–1577 (2018).
Roussos Torres, E. T. et al. Phase 1 study of entinostat and nivolumab with or without ipilimumab in advanced solid tumors (ETCTN-9844). Clin. Cancer Res. 27, 5828–5837 (2021).
Zou, Y. et al. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: a systematic review and meta-analysis. Ther. Adv. Med. Oncol. 12, 17588835920940928 (2020).
Hendry, S. et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv. Anat. Pathol. 24, 311–335 (2017).
Loupakis, F. et al. Prediction of benefit from checkpoint inhibitors in mismatch repair deficient metastatic colorectal cancer: role of tumor infiltrating lymphocytes. Oncologist 25, 481–487 (2020).
Hellmann, M. D. et al. Entinostat plus pembrolizumab in patients with metastatic NSCLC previously treated with anti-PD-(L)1 therapy. Clin. Cancer Res. 27, 1019–1028 (2021).
Zitvogel, L., Apetoh, L., Ghiringhelli, F. & Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 8, 59–73 (2008).
Bergenfelz, C. & Leandersson, K. The generation and identity of human myeloid-derived suppressor cells. Front. Oncol. 10, 109 (2020).
Angell, T. E. et al. Circulating myeloid-derived suppressor cells predict differentiated thyroid cancer diagnosis and extent. Thyroid 26, 381–389 (2016).
Lechner, M. G., Liebertz, D. J. & Epstein, A. L. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 185, 2273–2284 (2010).
Pan, P.-Y. et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 70, 99–108 (2010).
Ferrant, J., Lhomme, F., le Gallou, S., Irish, J. M. & Roussel, M. Circulating myeloid regulatory cells: promising biomarkers in B-cell lymphomas. Front. Immunol. 11, 3686 (2021).
Toor, S. M. et al. Myeloid cells in circulation and tumor microenvironment of breast cancer patients. Cancer Immunol. Immunother. 66, 753–764 (2017).
Sidiropoulos, D. N. et al. Entinostat decreases immune suppression to promote antitumor responses in a HER2+ breast tumor microenvironment. Cancer Immunol. Res. 10, 656–669 (2022).
Boutros, C. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13, 473–486 (2016).
Adams, S. et al. A multicenter phase II trial of ipilimumab and nivolumab in unresectable or metastatic metaplastic breast cancer: cohort 36 of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART, SWOG S1609). Clin. Cancer Res. 28, 271–278 (2022).
Shen, J., Chen, X., Wang, Z., Zhang, G. & Chen, W. Downregulation of CD40 expression contributes to the accumulation of myeloid-derived suppressor cells in gastric tumors. Oncol. Lett. 8, 775–780 (2014).
Kim, Y., Shin, Y. & Kang, G. H. Prognostic significance of CD103+ immune cells in solid tumor: a systemic review and meta-analysis. Sci. Rep. 9, 3808 (2019).
Cao, Q. et al. CD103+ dendritic cells elicit CD8+ T cell responses to accelerate kidney injury in adriamycin nephropathy. J. Am. Soc. Nephrol. 27, 1344–1360 (2016).
Zilio, S., Bicciato, S., Weed, D. & Serafini, P. CCR1 and CCR5 mediate cancer-induced myelopoiesis and differentiation of myeloid cells in the tumor. J. Immunother. Cancer 10, e003131 (2022).
Lee, Y. S. et al. Human CD141+ dendritic cells (cDC1) are impaired in patients with advanced melanoma but can be targeted to enhance anti-PD-1 in a humanized mouse model. J. Immunother. Cancer 9, e001963 (2021).
Campbell, J. J. et al. CCR7 expression and memory T cell diversity in humans. J. Immunol. 166, 877–884 (2001).
Ito, T. et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 204, 105–115 (2007).
Yang, C. et al. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/Bcl-2 signaling pathway. Med. Oncol. 32, 352 (2015).
DeLeeuw, R. J., Kost, S. E., Kakal, J. A. & Nelson, B. H. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin. Cancer Res. 18, 3022–3029 (2012).
Wei, S. C. et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 170, 1120–1133 (2017).
Taube, J. M. et al. Implications of the tumor immune microenvironment for staging and therapeutics. Mod. Pathol. 31, 214–234 (2018).
Cimino-Mathews, A. Tumor-infiltrating lymphocytes and PD-L1 in breast cancer (and, what happened to medullary carcinoma?). Diagn. Histopathol. 27, 148–154 (2021).
Jagannathan, G., White, M. J., Xian, R. R., Emens, L. A. & Cimino-Mathews, A. A new landscape of testing and therapeutics in metastatic breast cancer. Surg. Pathol. Clin. 15, 105–120 (2022).
Yarchoan, M., Hopkins, A. & Jaffee, E. M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 377, 2500–2501 (2017).
Barroso-Sousa, R. et al. Nimbus: a phase II study of nivolumab plus ipilimumab in metastatic hypermutated HER2-negative breast cancer. J. Clin. Oncol. 37, TPS1115 (2019).
Marcus, L. et al. FDA approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin. Cancer Res. 27, 4685–4689 (2021).
Barroso-Sousa, R. et al. Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann. Oncol. 31, 387–394 (2020).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Zhao, M. et al. A liquid chromatography/tandem mass spectrometry assay to quantitate MS-275 in human plasma. J. Pharm. Biomed. Anal. 43, 784–787 (2007).
Shin, S. M. et al. CyTOF protocol for immune monitoring of solid tumors from mouse models. STAR Protoc. 4, 101949 (2022).
Takada, K. et al. Use of the tumor-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to combination therapy with pertuzumab, trastuzumab, and docetaxel for advanced HER2-positive breast cancer. J. Transl. Med. 16, 86 (2018).
Sunshine, J. C. et al. PD-L1 expression in melanoma: a quantitative immunohistochemical antibody comparison. Clin. Cancer Res. 23, 4938–4944 (2017).
Kim, A. K. et al. Multiple immune-suppressive mechanisms in fibrolamellar carcinoma. Cancer Immunol. Res. 7, 805–812 (2019).
Denkert, C. et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 19, 40–50 (2017).
Dieci, M. V. et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on breast cancer. Semin. Cancer Biol. 52, 16–25 (2017).
Gaule, P. et al. A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol. 3, 256–259 (2017).
Rimm, D. L. et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non–small cell lung cancer. JAMA Oncol. 3, 1051–1058 (2017).
Kandoth, C. mskcc/vcf2maf: convert a VCF into a MAF, where each variant is annotated to only one of all possible gene isoforms. GitHub https://github.com/mskcc/vcf2maf (2021).
Mayakonda, A., Lin, D. C., Assenov, Y., Plass, C. & Koeffler, H. P. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 28, 1747–1756 (2018).
Acknowledgements
We thank the individuals who volunteered to participate in this study, NCI CTEP, Bristol Myers Squibb and Syndax Pharmaceuticals for the supply of study drugs via NCI and the research teams and physicians at participating sites. We thank C. Thornburn from the Johns Hopkins Immune Monitoring Core, G. Rosner and M. Carducci for valuable input during the study and S. Do from the University of Southern California for help finalizing the paper for publication. The following sources provided funding for the current study: NCI’s Experimental Therapeutics Clinical Trials Network (UM1CA186689, UM1CA186690, UM1CA186691, UM1CA186717 and U24CA247648 to E.T.R.T., R.M.C., V.S., E.J.F. and E.M.J.), Bloomberg Kimmel Institute for Immunotherapy (E.T.R.T. and E.M.J.), Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (P30CA006973, UL1TR 001079 and 1S10RR026824-01 to M.A.R.) and the Clinical Protocol and Data Management facilities (P30CA006973, P30CA014089 and P30CA047904 to A.O. and M.D.). The Cancer Therapy Evaluation Program supplied entinostat, nivolumab and ipilimumab. Support was also provided by the Commonwealth Foundation Grant (2018-2020 to E.T.R.T. and NCCN Young Investigator Award 2015 to R.M.C.), Mary Kay Foundation (Cancer Research Grant Program 2015 to V.S.), V Foundation (Translational Award 2017 to R.M.C. and V.S.), Tower Cancer Research Foundation (Career Development Award 2019–2021 to E.T.R.T.), Concern Foundation (Conquer Cancer Now Award 2020–2022 to E.T.R.T.), METAVivor (Young Investigator Award 2022–2024 to E.T.R.T.), Dana and Albert ‘Broccoli’ Professorship and the Broccoli Foundation and the Breast Cancer Research Foundation (to E.M.J.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
First corresponding author E.T.R.T. and senior corresponding author R.M.C. equally contributed to data collection and analysis along with other members of the team and formulated the whole paper. R.M.C. served as the lead principal investigator on the clinical trial until she moved to Ireland in 2019. E.T.R.T. served as the translational principal investigator. W.J.H. performed liquid-based CyTOF, analyzed the data and provided feedback on the manuscript. L.D. and J.A.T. provided statistical analyses for clinical and correlative results, provided text for statistical sections and edited the paper. J.L. and C.R. helped process, organize, prepare and deliver specimens for analyses. C.W. provided statistical support for initial clinical analysis. A.B., P.L.R., V.C. and Y.Y. served as site principal investigators during the clinical trial and provided feedback on the paper. M.D. served as the primary clinical research coordinator for the study. A.O. served as the main clinical regulatory supervisor for the trial. S.M.S. and A.H. provided technical assistance in running liquid-based CyTOF. E.L.E. optimized and performed IHC staining of tumor samples. R.P. and H.S. served as primary contacts with NCI/CTEP and provided input on the paper. Z.T. and M.A.R. performed the pharmacokinetic analyses. Q.Z. analyzed CD8 and FoxP3 staining, R.A.A. and A.C.-M. served as the primary study pathologists, and A.C.-M. scored PD-L1 and TILs on all samples and provided input on paper writing. E.J.F. provided senior input for statistical analysis and genomic correlative data. E.M.J. provided input on the clinical trial design and at all stages of enrollment, analysis and paper preparation. V.S. took over as the primary clinical principal investigator when R.M.C. moved to Ireland and also provided input on clinical trial design and at all stages of enrollment, analysis and paper preparation.
Corresponding authors
Ethics declarations
Competing interests
R.M.C. has received an unrestricted educational grant from Pfizer and research funding for clinical trials from MSD Ireland, Pfizer, Daiichi Sankyo and AstraZeneca, all to her institution. R.M.C. has consulted for AstraZeneca/Daichii, Gilead and Seagen and has received travel support from Novartis. M.A.R. has received research grants to the institution from Celgene, Cullinan Apollo and RenovoRx and is a cofounder and board member and holds equity in Geminus Therapeutics. M.A.R.’s spouse is employed by GlaxoSmithKline. V.S. received research grants to their institution from Abbvie, Biocept, Pfizer, Novartis and Puma Biotechnology and is a member of the Data Safety Monitoring Board at Immunomedics and AstraZeneca. A.C.-M. has received research grants to their institution from HeritX, Genentech and Bristol Myers Squibb and serves as a consultant to Bristol Myers Squibb. A.B. is a paid consultant for Lilly, Novartis, Pfizer, Astrazeneca, Eisai, Roche and Sanofi. P.L.R. reports personal fees from Abbvie, Agios, Five Prime, GenMab, Halozyme, Roche-Genentech, Genentech, Cytomx, Takeda, SOTIO, Cybrexa, Agenus, Tyme, IQVIA, TRIGR, Pfizer, ImmunoMet, Black Diamond, Glaxo-Smith Kline, QED Therapeutics, AstraZeneca, EMD Serono, Shattuck, Astellas, Salarius, Silverback, MacroGenics, Kyowa Kirin, Kineta, Zentalis, Molecular Templates, ABL Bio, SK Life Science and STCube; these are all outside the submitted work. E.M.J. reports ongoing collaboration with Roche on new approaches to Precision Medicine Tumor Boards. V.C. is part of the speaker’s bureau for Ipsen and Coherus and is a Perthera and Pfizer consultant. Y.Y. is part of the speaker’s bureau for Gilead, AstraZeneca, Daiichi Sankyo and Merck, is a paid consultant for Gilead, Pfizer, Stemline and AstraZeneca and has received research support from Genentech, Merck, Pfizer, Imugene, Agenus and Gateway Foundation. R.A.A. has received research support from Bristol Myers Squibb, Merck, StandUp2Cancer and FLXbio and is a paid consultant for Bristol Myers Squibb, Merck, Incyte, AstraZeneca and FLXbio. E.J.F. is on the Scientific Advisory Board of Resistance Bio/Viosera Therapeutics and is a paid consultant for Merck and Mestag Therapeutics. E.M.J. is a paid consultant for Adaptive Biotech, CSTONE, Achilles, DragonFly and Genocea. E.M.J. receives funding from Lustgarten Foundation and Bristol Myers Squibb and is the Chief Medical Advisor for Lustgarten and a Scientific Advisory Board member for Parker Institute for Cancer Immunotherapy and C3 Cancer Institute. E.M.J. reports other support from Abmeta and Adventris, personal fees from Achilles, Dragonfly, Mestag, The Medical Home Group and Surgtx, other support from Parker Institute and grants and other support from the Lustgarten Foundation, Genentech, Bristol Myers Squibb and Break Through Cancer outside the submitted work. E.T.R.T. is a consultant for Synaptical and is a shareholder in this company. All other authors have nothing to disclose.
Peer review
Peer review information
Nature Cancer thanks Dominic Edelmann, Heather McArthur and Hatem Soliman for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
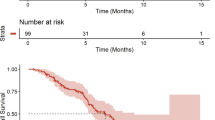
Extended Data Fig. 1 Survival evaluation in all 24 patients.
A. Progression-free Survival (PFS) including all 24 patients. B. Overall survival (OS) including all 24 patients. Dotted lines at 50% survival probability represent mean values listed for each graph and indicate PFS/ OS and 95% CIs. Abbreviations: CI, confidence interval; NA, not applicable.
Extended Data Fig. 2 Peripheral CyTOF for Immune Evaluation.
A. CyTOF expression of markers used to determine immune cell subsets. Cell sub types listed on the right were assigned based on differential expression of the markers listed across the bottom. Neu- neutrophil, GMDSC- granulocytic myeloid derived suppressor cell, MMDSC_I-III monocytic myeloid derived suppressor cell types I-III, Mono I-III- monocytes types I-III, cDC1- classical dendritic cell type 1, cDC2-classic dendritic cell type 2, pDC-plasmacytoid dendritic cell, Baso-basophil, CD4_T- CD4 positive T cell, CD8_T- CD8 positive T cell, DNT- double negative T cells (neither CD4 nor CD8 expressing T cells), DPT-double positive T cells (both CD4 and CD8 positive), NK_like-natural killer cell, B- B cell, UA- unassigned cell type. B. Expression of CCR5 at baseline and Post Run-in. C. Expression of CD141 at baseline and Post Run-in. D. Expression of HLADR at baseline and Post Run-in. All P values listed in associated tables represent two-sided paired Wilcoxon’s rank sum testing. *Denotes significant change sustained at Week 8. N = 17 at baseline, N = 17 at Post Run-in, and N = 10 at Week 8 biologically independent samples. Box plots show the median, upper, and lower quartiles, and whiskers extend to 1.5 times the interquartile range.
Extended Data Fig. 3 Peripheral CyTOF lymphocyte marker evaluation.
No significant differences in proportions of CD4 and CD8 T cell, B cell and NK cell populations across the treatment groups and in association with response (A). Significant changes in expression in markers on CD4 T cells (A) and CD8 T cells (B) in responders and non-responders (by RECIST). Values in orange represent a mean increase of marker expression at Post Run-In calculated as difference between baseline and Post Run-In (delta). Values in blue represent a mean decrease in at Post Run-In. *Indicates markers with sustained significant change between Run-In and Week 8. P values represent pairwise Wilcoxon rank sums. C. Expression of markers on B cells from peripheral CyTOF. Non-responders by RECIST (red), responders (blue). P values are indicative of two-sided pairwise Wilcoxon’s rank sum testing and compares the differences in value from baseline to Post Run-in in responders vs. non-responders listed in table. N = 17 at baseline, n = 17 at Post Run-in, and n = 10 at Week 8 biologically independent samples. Box plots show the median, upper, and lower quartiles, and whiskers extend to 1.5 times the interquartile range.
Extended Data Fig. 4 Evaluation of tumor biopsies for T cell infiltration.
Ratio of CD8/FoxP3. A. Log-transformed ratios of CD8 density to FoxP3 density at different timepoints. N = 12 at baseline, n = 10 at Post Run-in, and n = 4 at Week 8 biologically independent samples. B. Average TIL percentage change over time and by response. *In these cases, only tumor bed was noted (no tumor visualized) consistent with complete tumor regression. N = 17 at baseline, n = 17 at Post Run-in, and n = 8 at Week 8 biologically independent samples. P-value is from pairwise two-sided Wilcoxon’s rank sum test. Box plots show the median, upper, and lower quartiles, and whiskers extend to 1.5 times the interquartile range.
Extended Data Fig. 5 Evaluation of PD-L1 status.
A. Average percentage of immune cells demonstrating PD-L1 staining by response and by breast cancer subtype at baseline (n = 14 biologically independent samples). B. Average percentage of tumor cells demonstrating PD-L1 staining by response and by breast cancer subtype at baseline (n = 14 biologically independent samples). P-value is from pairwise two-sided Wilcoxon’s rank sum test. Red dots indicate HR-positive patients and blue indicate TNBC. Box plots show the median, upper, and lower quartiles, and whiskers extend to 1.5 times the interquartile range.
Extended Data Fig. 6 Mutational type analysis.
A. Summary of incidence of variant classifications across all samples. B. Summary of types of variants; triple nucleotide polymorphism (TNP), single nucleotide polymorphism (SNP), oligo-nucleotide polymorphism (ONP), insertion (INS), double nucleotide polymorphism (DNP), deletion (DEL). C. Single nucleotide variant (SNV) classes at baseline across all samples. T-thymine, G-guanine, A-adenosine, C-cytosine. For example thymine altered to guanine (T > G). D. Top 10 mutated genes in responders. E. Top 10 mutated genes in non-responders. Percentages listed next to each mutated gene indicates number of samples with mutations in given gene.
Supplementary information
Supplementary Data
Statistical Data for Supplementary Tables.
Supplementary Information
Full copy of the approved study protocol.
Source data
Source Data Figs. 2–4 and Extended Data Figs. 1–5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roussos Torres, E., Ho, W.J., Danilova, L. et al. Entinostat, nivolumab and ipilimumab for women with advanced HER2-negative breast cancer: a phase Ib trial. Nat Cancer (2024). https://doi.org/10.1038/s43018-024-00729-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43018-024-00729-w
- Springer Nature America, Inc.