Abstract
Cellobiose has received increasing attention in various industrial sectors, ranging from food and feed to cosmetics. The development of large-scale cellobiose applications requires a cost-effective production technology as currently used methods based on cellulose hydrolysis are costly. Here, a one-pot synthesis of cellobiose from sucrose was conducted using a recombinant Pichia pastoris strain as a reusable whole-cell biocatalyst. Thermophilic sucrose phosphorylase from Bifidobacterium longum (BlSP) and cellobiose phosphorylase from Clostridium stercorarium (CsCBP) were co-displayed on the cell surface of P. pastoris via a glycosylphosphatidylinositol-anchoring system. Cells of the BlSP and CsCBP co-displaying P. pastoris strain were used as whole-cell biocatalysts to convert sucrose to cellobiose with commercial thermophilic xylose isomerase. Cellobiose productivity significantly improved with yeast cells grown on glycerol compared to glucose-grown cells. In one-pot bioconversion using glycerol-grown yeast cells, approximately 81.2 g/L of cellobiose was produced from 100 g/L of sucrose, corresponding to 81.2% of the theoretical maximum yield, within 24 h at 60 °C. Moreover, recombinant yeast cells maintained a cellobiose titer > 80 g/L, even after three consecutive cell-recycling one-pot bioconversion cycles. These results indicated that one-pot bioconversion using yeast cells displaying two phosphorylases as whole-cell catalysts is a promising approach for cost-effective cellobiose production.
Similar content being viewed by others
Introduction
Cellobiose is a reducing disaccharide consisting of two glucose units linked through a β (1 → 4) glycosidic bond. It is a zero-calorie functional sweetener with 30% of the sweetness of sucrose, and does not cause an increase in blood glucose levels or insulin secretion when ingested1. It has attracted a lot of interest because of its biological functions2, including a soluble dietary fiber that promotes immunomodulatory health effects by influencing the bacterial composition of the human colonic microbiota3. In addition, the potential prebiotic effects of cellobiose have been demonstrated in animals, such as horses4 and rabbits5. Therefore, the development of a cost-effective large-scale cellobiose production method is required6.
Currently, industrial-scale production of cellobiose is primarily conducted by the enzymatic or acidic hydrolysis of cellulose. However, as the acidic hydrolysis of cellulose yields a complex mixture of oligosaccharides with varying degrees of polymerization, cellobiose yield is low, and its separation and purification are costly7. Enzymatic hydrolysis releases cellobiose by cleaving the second β (1 → 4) glycosidic bonds from reducing or nonreducing ends of cellulose mediated by cellobiohydrolase (CBH); however, digestion of cellulose requires high dosages of CBH8, hindering its application on a large scale.
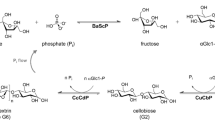
In recent years, there has been growing interest in the production of cellobiose from sucrose, an inexpensive industrial sugar9, using one-pot multi-enzyme biocatalysis as an alternative to cellulose hydrolysis. This process involves three enzymatic reactions: (1) the conversion of sucrose and phosphate to glucose-1-phosphate (G1P) and fructose, catalyzed by sucrose phosphorylase (SP; EC 2.4.1.7); (2) the conversion of fructose to glucose, catalyzed by xylose (glucose) isomerase (XI, EC 5.3.1.5); and (3) the conversion of G1P and glucose to cellobiose and phosphate, catalyzed by cellobiose phosphorylase (CBP; EC 2.4.1.20)10. The phosphate ions are recycled within the reaction system and maintained at a constant level (Fig. 1a). Kitaoka et al.10 achieved 50.3 g/L (147 mM) cellobiose production from 68.5 g/L (200 mM) sucrose in 100 h at 37 °C using this one-pot process. Recently, Zhong et al.11 reported 21.3 g/L (62.3 mM) cellobiose production from 34.2 g/L (100 mM) sucrose in 10 h using a one-pot process at 45 °C by employing thermostable SP, XI, and CBP. Increasing the reaction temperature offers multiple biomanufacturing advantages such as decreasing the risk of microbial contamination, decreasing viscosity for enhanced mass transfer, and faster reaction rates. However, further improvements in cellobiose yield and titer are required for economic feasibility. Moreover, although XI is inexpensively available as a commercial enzyme for food processing, the preparation of SP and CBP is costly; therefore, there is an urgent need to reduce the preparation cost of these phosphorylases.
Schematics of (a) one-pot synthesis of cellobiose from sucrose catalyzed by three enzymes and (b) one-pot synthesis of cellobiose from sucrose using yeast cells displaying two phosphorylases with commercial xylose isomerase. SP, sucrose phosphorylase (EC 2.4.1.7); XI, xylose isomerase (EC 5.3.1.5); CBP, cellobiose phosphorylase (EC 2.4.1.20); G1P, glucose-1-phosphate; Pi, inorganic phosphate.
The use of whole-cell biocatalysts, in which microbial cells express the required enzymes, is a promising approach for cost-effective multienzyme bioproduction processes. They are substantially cheaper to produce than free enzymes that require several steps for isolation and purification12. Easy separation and recovery of microbial cells from products makes recycling and reusing biocatalysts possible as long as cells maintain high enzyme activity for a long period of time12. Recent studies have explored whole-cell catalysis for cellobiose bioproduction from sucrose and glucose using recombinant Escherichia coli strains2,6. Wang et al.6 reported that 342 g/L (1.0 M) sucrose and 180 g/L (1.0 M) glucose were converted to 274 g/L (800 mM) cellobiose using SP and CBP co-expressing E. coli cells as whole-cell biocatalysts. However, their process left high concentrations of by-product fructose (∼180 g/L) and residual glucose (∼40 g/L), necessitating additional steps for removal such as yeast treatment to consume these sugars6. Furthermore, these studies did not assess the reusability of the constructed E. coli cells. To the best of our knowledge, one-pot synthesis of cellobiose from sucrose using reusable whole-cell biocatalysts has not yet been reported.
Recently, yeast cell-surface display technology has attracted attention as a promising approach for constructing reusable whole-cell biocatalysts with multiple enzymatic activities. Heterologous enzymes can be covalently anchored to the yeast cell wall by fusion with glycosylphosphatidylinositol (GPI)-cell wall proteins. Given that the anchored enzymes are exposed externally on the cell surface, the reaction rate is not constrained by mass transfer limitations across the cell membrane. However, this technology has not yet been applied to the one-pot synthesis of cellobiose from sucrose.
In this study, we reported the development of a recombinant yeast strain that efficiently displays two thermophilic phosphorylases (SP and CBP) on its cell surface via a GPI-anchoring system and one-pot synthesis of cellobiose from sucrose at elevated temperature using recombinant yeast cells as whole-cell biocatalysts (Fig. 1b). The Crabtree-negative yeast Pichia pastoris (Komagataella phaffii), widely used for heterologous protein production13 and highly suitable for cell-surface display technology14, was used as a host for the construction of whole-cell catalysts. We also evaluated the effect of the carbon source of the P. pastoris strain on cell-surface enzyme activity and cell titer. Finally, repetitive one-pot cellobiose synthesis was conducted by recycling SP and CBP co-displaying P. pastoris cells to investigate the reusability of yeast cells in a continuous process.
Results
Construction of SP-displaying P. pastoris strains
It is important to select an appropriate GPI-anchoring domain for each enzyme to maximize its activity on the yeast cell surface. In this study, the GPI-cell wall proteins GCW30, 34, 51, and 6115, which showed high cell-surface activity of cellulases in our previous study14, were used as GPI-anchoring domains for the cell-surface display of phosphorylases. All expression cassettes were constructed under the control of SPI1 promoter, a constitutive promoter in P. pastoris16. First, the expression cassettes of Bifidobacterium longum sucrose phosphorylase (BlSP) fused with GPI-anchoring domains were integrated into the P. pastoris CBS7435 genome, and the cell-surface SP activity of the constructed strains was evaluated as described in the Methods. The highest SP activity was observed when GCW30 was used as the GPI-anchoring domain (Fig. 2a).
Comparison of cell-surface enzyme activity of recombinant P. pastoris strains after cultivation in YPD medium for 48 h. (a) SP activity of SP displaying strains with different GPI-anchoring domains. (b) SP activity of recombinant strains expressing only BlSP display construct (Pp-SP-SSG30) and co-expressing BlSP display and secretion constructs (Pp-SP2). (c) CBP activity in strains co-displaying SP and CBP with different GPI-anchoring domains.
Improving cell-surface SP activity by co-expression of BlSP display and secretion constructs
Biologically active SP from Bifidobacterium adolescentis, which shares 93% identity with BlSP, exists as a homodimer17. Therefore, we hypothesized that BlSP activity is maximized through homodimerization. A surface-displayed protein can be dimerized by co-expression with a secreted protein18. For efficient dimerization of surface-displayed BlSP, a secretion construct of BlSP was expressed in the Pp-SP-SSG30 strain, and the SP activity of the resulting strain (Pp-SP2) was evaluated. As shown in Fig. 2b, cell-surface SP activity was significantly improved by the additional secretory expression of BlSP. The Pp-SP2 strain exhibited approximately 3.3-fold higher cell surface SP activity than the Pp-SP-SSG30 strain.
Construction of SP and CBP co-displaying P. pastoris strains
Clostridium stercorarium cellobiose phosphorylase (CsCBP), which is responsible for the final step in the conversion of sucrose to cellobiose, was additionally displayed on the cell surface of Pp-SP2. This enzyme acts as a monomer19. Gene cassettes for the expression of CsCBP fused with GCW30, 34, 51, and 61 anchoring domains were integrated into the Pp-SP2 genome. The cell-surface CBP activity of the constructed strains was evaluated. Among these strains, Pp-SP2CBP30, which uses GCW30 as the GPI-anchoring domain of CsCBP, showed relatively high CBP activity (Fig. 2c). Therefore, this strain was used for subsequent experiments.
One-pot synthesis of cellobiose from sucrose using SP and CBP co-displaying P. pastoris
One-pot synthesis of cellobiose from sucrose was conducted using P. pastoris strain displaying BlSP and CsCBP with commercial thermophilic XI at 60 °C for 24 h as described in the Methods. To evaluate the effects of carbon sources (glucose and glycerol) on cell titer and cellobiose productivity, the Pp-SP2CBP30 strain was cultivated in YPD or YPG media. The obtained cells were used for the one-pot synthesis of cellobiose from sucrose at an initial concentration of 100 g wet cells/L. After 48 h of cultivation in YPD medium, the cell titer of the Pp-SP2CBP30 strain was 1686 mg dry cells/L, and the SP and CBP activities of these cells were 18.4 and 18.9 U/g dry cells, respectively (Table 1). This strain produced approximately 40 g/L cellobiose from 100 g/L sucrose within 24 h of one-pot bioconversion (Fig. 3a). Alternatively, in YPG medium, the cell titer of the Pp-SP2CBP30 strain reached 2997 mg dry cells/L after a 48-h cultivation, and the SP and CBP activities were 60.0 and 35.5 U/g dry cells, respectively (Table 1). In the one-pot bioconversion using glycerol-grown yeast cells, 100 g/L sucrose was almost completely consumed and approximately 81.2 g/L cellobiose was produced within 24 h (Fig. 3b). Given that sucrose and cellobiose have the same molecular weight (342.3 g/mol), the cellobiose titer corresponded to 81.2% of the theoretical maximum yield. Approximately 7.1 g/L (39 mM) each of fructose and glucose, the intermediate sugars for this conversion, remained in the reaction system. The total C-mol conversion rate including these intermediate sugars was 94.5%.
Time course of one-pot synthesis of cellobiose from sucrose using SP and CBP co-displaying P. pastoris strain grown in, (a) YPD and (b) YPG media. Yeast cells were inoculated into the reaction mixture at an initial concentration of 100 g wet cells/L, and reaction was performed at 60 °C for 24 h while rotating at 35 rpm.
Repetitive cellobiose synthesis via yeast cell recycling
To investigate the reusability of SP and CBP co-displaying P. pastoris strain in a continuous process, repetitive cellobiose synthesis was conducted by recycling yeast cells (Fig. 4a). Cells of the Pp-SP2CBP30 strain grown in glycerol were collected by centrifugation and resuspended in fresh reaction mixture containing XI at the beginning of each run. In the first batch, 100 g/L sucrose was converted to 83.8 g/L cellobiose. The second batch yielded 86.5 g/L cellobiose, including that carried over from the first batch. In the third batch, although a significant decrease in sucrose consumption and cellobiose production rates was observed, the cellobiose concentration after a 24-h reaction was maintained at 81.0 g/L (Fig. 4b).
Discussion
Cellobiose is an important functional food additive, and an urgent need exists to establish a cost-effective bioprocess for its production. In this study, we demonstrated a one-pot synthesis of cellobiose from sucrose, an inexpensive industrial sugar, catalyzed by three thermophilic enzymes, using a whole-cell biocatalyst. Two thermophilic phosphorylases (BlSP and CsCBP) fused with an appropriate GPI-anchoring domain (GCW30) were produced by a recombinant P. pastoris strain and efficiently immobilized on its cell surface. The cell-surface SP activity of the recombinant P. pastoris strain significantly improved via the additional secretory expression of BlSP (Fig. 2b), suggesting facilitated homodimerization of cell-surface anchored BlSP. Utilizing yeast cells co-displaying SP and CBP (Pp-SP2CBP30) with commercial thermophilic XI, approximately 81.2 g/L (237 mM) cellobiose was produced from 100 g/L (292 mM) sucrose after 24 h of one-pot bioconversion at 60 °C (Fig. 3b), which corresponding to 81.2% of the theoretical maximum yield. The cellobiose yield and titer exceeded those of previously reported one-pot cellobiose production via SP, XI, and CBP [50.3 g/L (147 mM) cellobiose production from 68.5 g/L (200 mM) sucrose corresponding to 73.5% of the theoretical maximum yield10 and 21.3 g/L (62.3 mM) cellobiose production from 34.2 g/L (100 mM) sucrose corresponding to 62.3% of the theoretical maximum yield11]. The total C-mol conversion rate including intermediate sugars (fructose and glucose) was 94.5% (Fig. 3b), which indicates that the recombinant yeast cells displaying BlSP and CsCBP did not consume almost any of the sugars present in the reaction system and functioned only as biocatalysts for conversion of sucrose to cellobiose. Moreover, the recombinant yeast cells maintained a cellobiose titer > 80 g/L, even after repeating three consecutive cell-recycling one-pot bioconversion cycles (Fig. 4b), indicating its high reusability as a whole-cell biocatalyst. To our knowledge, this is the first study on the one-pot synthesis of cellobiose from sucrose using a reusable whole-cell catalyst.
In this study, the effect of a carbon source on the performance of a recombinant P. pastoris strain as a whole-cell biocatalyst was demonstrated. When Pp-SP2CBP30 cells were grown on glycerol, their cell-surface SP and CBP activities per cell weight increased by 3.3- and 1.9-fold, respectively, compared to glucose-grown cells (Table 1). Consequently, the cellobiose titer after a 24-h one-pot reaction almost doubled (Fig. 3). Furthermore, changing the carbon source from glucose to glycerol positively influenced cell titer. When cells were grown on glycerol, the mean cell titer was approximately 1.8-fold higher than when grown on glucose (Table 1). These results suggested that glycerol is a desirable carbon source for the mass production of recombinant P. pastoris cells as a high-performance whole-cell catalyst. Glycerol is a useful carbon source for P. pastoris to allow for higher biomass yield and elevated levels of heterologous proteins than those of glucose20,21. In addition, it has been reported that glycerol was the preferred carbon source compared to glucose for the expression of a heterologous protein (human lysozyme) in P. pastoris under the control of SPI1 promoter22, which was used for all expression cassettes in this study. These previous reports align with our present results. The marked increase in SP activity compared to that of CBP may be due to the homodimerization of surface-displayed BlSP facilitated by the increased secretory expression of BlSP.
Another possible factor for the improved performance of recombinant yeast cells using glycerol as a carbon source is the reduction in cell size. Cell size of P. pastoris varies depending on the type of carbon source, and the size of cells grown on glycerol is smaller than that of cells grown on glucose23. Supplementary Fig. S1 shows the microscopic images of Pp-SP2CBP30 cells grown for 48 h in media containing glucose (YPD) and glycerol (YPG). Although cell shapes were similar for both carbon sources, their sizes were statistically different. The mean diameter of cells grown on glycerol (6.1 ± 0.6 μm) was approximately 0.8 times that of cells grown on glucose (7.6 ± 0.9 μm). As the surface area per unit volume is inversely proportional to the diameter of an object, cells grown on glycerol exhibited approximately 1.25 times more surface area per unit volume of than those grown on glucose. In yeast cell-surface display technology, the cell surface serves as a crucial site for immobilizing enzymes via GPI-anchoring domain. The expanded cell surface area may increase the enzyme-displaying capacity of P. pastoris, resulting in improved SP and CBP activities per unit cell volume.
Several studies on one-pot bioconversion with multiple enzymes have shown that optimization of the activity ratio of enzymes is important for efficient bioconversion2,24,25. To further improve cellobiose production rate and yield in one-pot bioconversion using a recombinant P. pastoris strain, optimize activity ratios of SP, XI, and CBP is essential. Yeast cell-surface display technology allows control of enzyme activity ratios via selection of GPI-anchoring domain to be fused to target enzymes and gene cassette copy number integrated into the yeast genome26. If future studies can optimize the activity ratios of SP, XI, and CBP using a similar approach, it has the potential to make this bioconversion process even more cost-effective. Furthermore, optimization of the reaction time for each batch of cell-recycling one-pot cellobiose synthesis is also required. In the third batch of the repetitive cellobiose synthesis demonstrated in this study (Fig. 4), approximately 12 g/L of sucrose remained after the 24 h reaction, and more cellobiose could have been produced by extending the reaction time. Conversely, in the first and second batches, the conversion rate from sucrose to cellobiose in the early phase was faster, and the conversion reaction may have reached equilibrium earlier than 24 h. If the cellobiose productivity per unit time of the cell-recycling one-pot bioconversion could be maximized by optimizing the reaction time of each batch depending on the condition of whole-cell biocatalysts, economic feasibility of the process would be further improved.
Conclusion
In this study, we demonstrated, for the first time, a one-pot cellobiose synthesis from sucrose using three thermophilic enzymes (SP, XI, and CBP) with a whole-cell biocatalyst constructed using yeast cell surface display technology. Using glycerol-grown P. pastoris cells co-displaying BlSP and CsCBP in the one-pot bioconversion, approximately 81 g/L of cellobiose was produced from 100 g/L of sucrose within 24 h at 60 °C. The recombinant yeast cells maintained a cellobiose titer of > 80 g L even after three consecutive one-pot reactions at 60 °C, indicating its high reusability as a whole-cell biocatalyst for bioproduction. Although further research is needed to determine its economic effectiveness when compared to existing technologies such as cell-free enzyme-based systems, these results indicated that one-pot bioconversion using yeast cells displaying the two phosphorylases as whole-cell biocatalysts is a promising approach for cost-effective cellobiose production.
Methods
Microorganisms and media
The characteristics of all yeast strains used in this study are summarized in Table 2. All yeast transformants were derived from the P. pastoris wild type strain CBS7435, and were cultivated in YPD [10 g/L yeast extract, 20 g/L Bacto-peptone (Gibco, MI, USA), and 20 g/L glucose] or YPG [10 g/L yeast extract, 20 g/L Bacto-peptone, and 20 g/L glycerol] medium. E. coli strain DH5α (Toyobo, Osaka, Japan) was used for construction and amplification of plasmid DNA. The medium for E. coli growth was prepared as previously described27.
Plasmid construction and P. pastoris transformation
The plasmids and primers used in this study are listed in Table 2 and Supplementary Table S1, respectively. Detailed construction procedures for plasmids and P. pastoris strains are provided in Supplementary Methods.
Preparation of enzyme-displaying yeast cells
P. pastoris strains were pre-cultured in 5 mL of YPD or YPG media in test tubes at 30 °C and 200 rpm for 18 h, inoculated in 50 mL of YPD or YPG media in 100 mL Erlenmeyer flasks without baffles closed with silicone plugs (Type T-28, Silicosen; Shin-Etsu Polymer Co., Ltd., Tokyo, Japan) at an initial OD600 of 0.05, and cultivated microaerobically at 30 °C and 150 rpm for 48 h in a BR-43FL shaker incubator (Taitec, Saitama, Japan). The cells were collected by centrifugation at 1000 × g for 5 min and washed twice with distilled water. The washed cell pellets were resuspended in distilled water to 500 g/L, treated at 60 °C for 90 min to arrest glucose uptake, and used in subsequent experiments.
Enzyme assays
The cell-surface enzyme activities of SP and CBP were determined in the direction of sucrose and cellobiose phosphorolysis, respectively. The reaction mixture contained 100 g/L sucrose (SP), 100 g/L cellobiose (CBP), 80 mM sodium phosphate buffer (pH 7.0), and 100 g wet cells/L yeast cell suspension. The reaction was performed at 60 °C for 30 min while rotating at 35 rpm using a heat block (Thermo Block Rotator SN-06BN; Nissin, Tokyo, Japan), and the fructose and glucose concentrations in the supernatant were quantified using high-performance liquid chromatography (HPLC) as described below. One unit of SP and CBP activities were defined as the activity that released 1 μmol of fructose and glucose per min, respectively.
One-pot synthesis of cellobiose from sucrose
The reaction mixture contained 100 g/L sucrose, 80 mM sodium phosphate buffer (pH 7.0), 5 g/L MgSO4, 1.5 IU/mL xylose isomerase (Godo-Shusei, Hyogo, Japan), and 100 g wet cells/L yeast cell suspension. The reaction was performed at 60 °C for 24 h while rotating at 35 rpm using a heat block (Thermo Block Rotator SN-06BN). The reaction mixture was sampled at 0, 4, 8, and 24 h, and sucrose, glucose, fructose, and cellobiose concentrations in each sample were determined using HPLC (Shimadzu, Kyoto, Japan) equipped with a Sugar-D column (4.6 × 250 mm; Cosmosil, Nacalai Tesque, Kyoto, Japan) and an RID-10A refractive index detector (Shimadzu). The column was maintained at 30 °C, and acetonitrile/H2O (70:30) was used as the mobile phase at a flow rate of 1.0 mL/min.
In the cell-recycle cellobiose production, after the 24-h reaction described above, cells were collected by centrifugation at 1000 × g for 5 min. The pelleted cells were resuspended in fresh reaction mixture containing 100 g/L sucrose, 80 mM sodium phosphate buffer (pH 7.0), 5 g/L MgSO4, and 1.5 IU/mL xylose isomerase to 100 g wet cells/L. The reaction was repeated thrice sequentially.
Microscopic observation and size comparison of yeast cells
Microscopic images of P. pastoris cells in samples collected after 48 h of growth in YPD or YPG medium were obtained using a BZ-X810 microscope (Keyence, Osaka, Japan) equipped with apochromatic objective lenses. Cells in each bright-field image were identified and segmented using YeastSpotter28. The diameters of the segmented cells were measured in a masked manner using ImageJ software (version 1.54d; NIH, Bethesda, MD, USA). To remove possible detection errors, the candidate cells were screened based on size (> 10 μm2) and circularity (> 0.5). Significance was determined using a two-tailed Student’s t-test; n = 79 (YPD) and 90 (YPG) cells.
Statistical analyses
Data from enzyme assays and one-pot synthesis of cellobiose are presented as the mean ± standard deviation of three independent experiments. Significant differences between groups were calculated using a two-tailed Student’s t-test. Differences with a confidence level of 95% (p < 0.05) were considered significant. All analyses were performed using Excel software (Microsoft-365).
Data availability
All data generated or analyzed during this study were included in this published article.
References
Nakamura, S., Oku, T. & Ichinose, M. Bioavailability of cellobiose by tolerance test and breath hydrogen excretion in humans. Nutrition 20, 979–983. https://doi.org/10.1016/j.nut.2004.08.005 (2004).
Schwaiger, K. N. et al. Plasmid design for tunable two-enzyme co-expression promotes whole-cell production of cellobiose. Biotechnol. J. 15, e2000063. https://doi.org/10.1002/biot.202000063 (2020).
Collins, M. D. & Gibson, G. R. Probiotics, prebiotics, and synbiotics: Approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 69, 1052S-1057S. https://doi.org/10.1093/ajcn/69.5.1052s (1999).
Paßlack, N., Vahjen, W. & Zentek, J. Impact of dietary cellobiose on the fecal microbiota of horses. J. Equine Vet. Sci. 91, 103106. https://doi.org/10.1016/j.jevs.2020.103106 (2020).
Ocasio-Vega, C. et al. Effect of cellobiose supplementation on growth performance and health in rabbits. Livest. Sci. 221, 163–171. https://doi.org/10.1016/j.livsci.2019.02.002 (2019).
Wang, L., Zheng, P., Hu, M. & Tao, Y. Inorganic phosphate self-sufficient whole-cell biocatalysts containing two co-expressed phosphorylases facilitate cellobiose production. J. Ind. Microbiol. Biotechnol. 49, kuac008. https://doi.org/10.1093/jimb/kuac008 (2022).
Zhang, Y. H. & Lynd, L. R. Cellodextrin preparation by mixed-acid hydrolysis and chromatographic separation. Anal. Biochem. 322, 225–232. https://doi.org/10.1016/j.ab.2003.07.021 (2003).
Ubiparip, Z., Moreno, D. S., Beerens, K. & Desmet, T. Engineering of cellobiose phosphorylase for the defined synthesis of cellotriose. Appl. Microbiol. Biotechnol. 104, 8327–8337. https://doi.org/10.1007/s00253-020-10820-8 (2020).
Jiang, W., Zhao, J., Wang, Z. & Yang, S. T. Stable high-titer n-butanol production from sucrose and sugarcane juice by Clostridium acetobutylicum JB200 in repeated batch fermentations. Bioresour. Technol. 163, 172–179. https://doi.org/10.1016/j.biortech.2014.04.047 (2014).
Kitaoka, M., Sasaki, T. & Taniguchi, H. Conversion of sucrose into cellobiose using sucrose phosphorylase, xylose isomerase and cellobiose phosphorylase. Denpun Kagaku 39, 281–283. https://doi.org/10.5458/jag1972.39.281 (1992).
Zhong, C., Wei, P. & Zhang, Y. H. P. A kinetic model of one-pot rapid biotransformation of cellobiose from sucrose catalyzed by three thermophilic enzymes. Chem. Eng. Sci. 161, 159–166. https://doi.org/10.1016/j.ces.2016.11.047 (2017).
de Carvalho, C. C. Whole cell biocatalysts: Essential workers from Nature to the industry. Microb. Biotechnol. 10, 250–263. https://doi.org/10.1111/1751-7915.12363 (2017).
Yang, Z. & Zhang, Z. Engineering strategies for enhanced production of protein and bio-products in Pichia pastoris: A review. Biotechnol. Adv. 36, 182–195. https://doi.org/10.1016/j.biotechadv.2017.11.002 (2018).
Inokuma, K. et al. Direct production of 4-hydroxybenzoic acid from cellulose using cellulase-displaying Pichia pastoris. Biotechnol. Bioeng. 120, 1097–1107. https://doi.org/10.1002/bit.28321 (2023).
Zhang, L. et al. Screening for glycosylphosphatidylinositol-modified cell wall proteins in Pichia pastoris and their recombinant expression on the cell surface. Appl. Environ. Microbiol. 79, 5519–5526. https://doi.org/10.1128/AEM.00824-13 (2013).
Ata, Ö., Prielhofer, R., Gasser, B., Mattanovich, D. & Çalık, P. Transcriptional engineering of the glyceraldehyde-3-phosphate dehydrogenase promoter for improved heterologous protein production in Pichia pastoris. Biotechnol. Bioeng. 114, 2319–2327. https://doi.org/10.1002/bit.26363 (2017).
van den Broek, L. A. et al. Physico-chemical and transglucosylation properties of recombinant sucrose phosphorylase from Bifidobacterium adolescentis DSM20083. Appl. Microbiol. Biotechnol. 65, 219–227. https://doi.org/10.1007/s00253-003-1534-x (2004).
Burns, M. L. et al. Directed evolution of brain-derived neurotrophic factor for improved folding and expression in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 80, 5732–5742. https://doi.org/10.1128/AEM.01466-14 (2014).
Reichenbecher, M., Lottspeich, F. & Bronnenmeier, K. Purification and properties of a cellobiose phosphorylase (CepA) and a cellodextrin phosphorylase (CepB) from the cellulolytic thermophile Clostridium stercorarium. Eur. J. Biochem. 247, 262–267. https://doi.org/10.1111/j.1432-1033.1997.00262.x (1997).
Gao, M. J. & Shi, Z. P. Process control and optimization for heterologous protein production by methylotrophic Pichia pastoris. Chinese J. Chem. Eng. 21, 216–226. https://doi.org/10.1016/S1004-9541(13)60461-9 (2013).
Peña, D. A., Gasser, B., Zanghellini, J., Steiger, M. G. & Mattanovich, D. Metabolic engineering of Pichia pastoris. Metab. Eng. 50, 2–15. https://doi.org/10.1016/j.ymben.2018.04.017 (2018).
Cankorur-Cetinkaya, A. et al. Process development for the continuous production of heterologous proteins by the industrial yeast. Komagataella phaffii. Biotechnol. Bioeng. 115, 2962–2973. https://doi.org/10.1002/bit.26846 (2018).
Diaz Arias, C. A. et al. Influence of carbon source on cell size and production of anti LDL (-) single-chain variable fragment by a recombinant Pichia pastoris strain. Mol. Biol. Rep. 46, 3257–3264. https://doi.org/10.1007/s11033-019-04785-9 (2019).
Chen, H., Huang, R. & Zhang, Y. P. Systematic comparison of co-expression of multiple recombinant thermophilic enzymes in Escherichia coli BL21(DE3). Appl. Microbiol. Biotechnol. 101, 4481–4493. https://doi.org/10.1007/s00253-017-8206-8 (2017).
Otte, K. B., Kittelberger, J., Kirtz, M., Nestl, B. M. & Hauer, B. Whole-cell one-pot biosynthesis of azelaic acid. Chemcatchem 6, 1003–1009. https://doi.org/10.1002/cctc.201400103 (2014).
Inokuma, K., Yoshida, T., Ishii, J., Hasunuma, T. & Kondo, A. Efficient co-displaying and artificial ratio control of α-amylase and glucoamylase on the yeast cell surface by using combinations of different anchoring domains. Appl. Microbiol. Biotechnol. 99, 1655–1663. https://doi.org/10.1007/s00253-014-6250-1 (2015).
Inokuma, K., Hasunuma, T. & Kondo, A. Ethanol production from N-acetyl-d-glucosamine by Scheffersomyces stipitis strains. AMB Express 6, 83. https://doi.org/10.1186/s13568-016-0267-z (2016).
Lu, A. X., Zarin, T., Hsu, I. S. & Moses, A. M. YeastSpotter: accurate and parameter-free web segmentation for microscopy images of yeast cells. Bioinformatics 35, 4525–4527. https://doi.org/10.1093/bioinformatics/btz402 (2019).
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
K.I. mainly performed the experiments and wrote the manuscript. K.I., K.T., T. Hamada, and T. Hasunuma conceived and designed the experiments. K.T., T. Hamada, and T. Hasunuma revised the manuscript for important intellectual content. K.T. and A.K. conceived and supervised the research. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Inokuma, K., Toyohara, K., Hamada, T. et al. One-pot synthesis of cellobiose from sucrose using sucrose phosphorylase and cellobiose phosphorylase co-displaying Pichia pastoris as a reusable whole-cell biocatalyst. Sci Rep 14, 18540 (2024). https://doi.org/10.1038/s41598-024-69676-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69676-x
- Springer Nature Limited