Abstract
This work presents experimental results on the energy efficiency in hydrogen production using atmospheric microwave plasma (915 MHz) through steam reforming of ethanol. Ethanol was chosen as a liquid hydrogen carrier due to its high hydrogen atom content, low cost, and wide availability. The experimental work began with the maximization of an energy efficiency of the used microwave plasma source. The process of maximization involved determining a position of a movable plunger that ensures the most efficient transfer of microwave energy from a microwave source to the generated plasma in the microwave plasma source. The aim of the investigations was to test the following working conditions of the microwave plasma source: absorbed microwave power PA by the generated plasma (up to 5.4 kW), the carrier gas volumetric flow rate (up to 3900 Nl/h), and the amount of the introduced ethanol vapours on the efficiency of hydrogen production (up to 2.4 kg/h). In the range of tested working conditions, the highest energy yield for hydrogen production achieved a rate of 26.9 g(H2)/kWh, while the highest hydrogen production was 99.3 g(H2)/h.
Similar content being viewed by others
Introduction
The landscape of global energy is currently dominated by fossil fuels, contributing to approximately 80% of the world’s energy1,2. For decades, fossil fuels such as crude oil, natural gas, and coal have been the primary means of energy production. However, the combustion of these fossil fuels comes at a significant environmental cost. The combustion process releases several by-products that have detrimental effects on both the natural environment and human health. The most concerning by-products include carbon dioxide (CO2), carbon monoxide (CO), sulphur dioxide (SO2), nitrogen oxides (NOx), particulate matter and heavy metals.
The natural concentration of carbon dioxide in the Earth’s atmosphere is approximately 0.04%3,4. However, due to rapid industrialisation, elevated levels of CO2 have been observed, contributing to global climate change. Carbon monoxide is a toxic gas that is both colourless and odourless5,6. Exposure to this gas can lead to tissue hypoxia, which poses severe health risks. Sulphur dioxide6 is toxic to both animals and humans and contributes to the formation of acid rain and urban smog. Nitrogen oxides6, mainly nitrogen dioxide, can cause respiratory issues and contribute to air pollution. Particulate matter6, which is composed of fine-grained dust, can pose a risk to respiratory health, causing lung diseases and even cancer. Additionally, heavy metals like lead, radium, and thorium, can also pose serious health risks when released into the atmosphere7. The environmental impact of these by-products has led to a global need to transition away from fossil fuel based economies, particularly in the energy sector. The depletion of fossil fuel reserves, coupled with the oil crisis in the 1970s8, highlighted the importance of finding new, sustainable, and environmentally friendly energy sources.
In response to the need for future energy sources, three main options are proposed: renewable energy, nuclear power, and clean coal technologies. The ‘hydrogen economy’ can complement each of these options by serving hydrogen as an energy carrier, while fuel cells convert the stored energy in hydrogen molecules into electricity9,10,11,12,13. This concept has garnered attention especially in the automotive industry, where hydrogen fuel cells are considered a viable alternative for traditional combustion engines.
The transition from fossil fuels to the hydrogen economy is motivated by: energy source diversification, environmental protection, and price stability. Hydrogen occurs in abundance in many of the earth’s compounds, offering many opportunities for its production, depending on the local sources available. The most significant current issue related to hydrogen production is the reduction of its production cost. The U.S. Department of Energy (DOE)13,14 conducted a comprehensive assessment of hydrogen policy, aiming for economically viable technologies with minimal greenhouse gas emissions. To analyse the future development of the hydrogen economy, it is convenient to divide the hydrogen production system into two categories13,14:
-
the centralised hydrogen production system, i.e. large scale hydrogen production technologies;
-
the decentralised (distributed) hydrogen production system, i.e. small and medium scale hydrogen production technologies.
According to the DOE, the accepted industrial cost of hydrogen production is 2 USD/kg(H2), equivalent to 60 g(H2)/kWh, the so-called target for 2030. The centralised hydrogen production system already meets this criterion. However, this criterion is fulfilled by steam reforming of natural gas, which involves releasing CO2 into the atmosphere and is dependent on fossil fuels. In addition, the centralised system requires a suitable infrastructure to deliver the hydrogen to the end user. An alternative solution is to produce hydrogen close to the user. Unfortunately, the methods used in centralised hydrogen production are not easily scalable down due to the use of catalysts and high temperatures. Reducing the production scale results in an increase in the cost of the obtained hydrogen. Therefore, there is a need for an efficient but inexpensive method of hydrogen production on a small or medium scale, close to the end-user. The distributed hydrogen production system avoids costs of hydrogen storage and transport. It is not intended to replace the centralized hydrogen production system, but instead, it is expected to be a significant complement to it in the future.
Currently, methods of distributed hydrogen production do not meet the DOE’s criterion of 60 g(H2)/kWh. Moreover, many methods of hydrogen production in the distributed system have the drawback of releasing large amounts of CO2 into the atmosphere. The CO2 is released into the atmosphere directly during the hydrogen production process and indirectly during the generation of electrical energy necessary for the hydrogen production process. This situation creates a paradox where hydrogen as an energy carrier does not contribute to CO2 emissions into the atmosphere, but the processes leading to its production do.
One of the potential methods for decentralised hydrogen production is the plasma method15,16,17,18,19,20. In this method, the plasma generated by electrical discharge converts hydrogen carriers (substances containing hydrogen atoms) into hydrogen. The catalytic properties of plasma result from the presence of high-energy electrons in the plasma, which induce the formation of chemically active particles and radicals21. The DOE scenario does not foresee a significant role for plasma technologies in the global hydrogen economy. However, the costs of hydrogen obtained through currently proposed methods for distributed hydrogen production are insufficient to be implemented in the industry. To meet the demand for cost-effective small-scale hydrogen production methods, it is essential to explore new, efficient, and primarily economical approaches. Accordingly, researchers are investigating the use of microwave plasma as a means of generating hydrogen.
The main objective of this work is to gather experimental results regarding the effectiveness of microwave plasma as a method for hydrogen production from conversion of ethanol. Ethanol is an attractive source for hydrogen production due to its high hydrogen atom content, low cost, and wide availability, derived from renewable sources. Its utilisation not only supports sustainable energy practices but also contributes to the development of green technologies. A unique aspect of this work is the exploration of hydrogen production by steam reforming process using plasma generated at the rarely encountered microwave frequency of 915 MHz. The use of 915 MHz microwaves for plasma generation is a relatively less common technique compared to the better known 2.45 GHz frequency. The main advantages of using 915 MHz compared to 2.45 GHz microwaves lie in its deeper penetration depth in the plasma and higher power capabilities. The advantages results in more efficient heating and processing of plasmo-chemical reactions, which is advantageous for applications requiring uniform energy distribution throughout the plasma volume. Additionally, the larger size of 915 MHz equipment allows for higher power capabilities, making it suitable for industrial applications requiring higher power levels. Furthermore, our research is conducted under atmospheric pressure conditions, which makes the presented method more applicable to industrial processes.
The microwave plasma source (MPS) is a device for generating low-temperature microwave plasma at atmospheric pressure21. The experimental investigations began with the maximization of the energy efficiency of the MPS. The process of maximization involved determining the position of a movable plunger that ensures the most efficient transfer of microwave energy from the microwave source to the generated plasma in the MPS. This process was supported by computer simulations of the distribution of the electric field module |E| within the MPS. Next, tests were conducted to investigate the microwave energy efficiency of hydrogen production from ethanol vapour for the determined position of the movable plunger. The aim was to evaluate the effect of different working conditions of the MPS (include: the absorbed microwave power PA, the carrier gas flow rate and the amount of ethanol vapour introduced) on the efficiency of hydrogen production.
Experimental setup
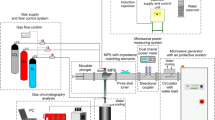
Diagram of the experimental setup is presented in Fig. 1. The setup consists of a microwave generator, a waveguide for microwave transmission, the MPS, and an installation for supplying the working gas (carrier gas + hydrogen carrier in the form of vapours) to the plasma source22. A photograph of the setup is shown in Fig. 2.
The microwave generator comprises the following components: a high-voltage power supply, a control panel, and a magnetron. It enables the generation of 915 MHz microwave frequency within a power range of up to 20 kW. The waveguide for microwave transmission is constructed using standard waveguide WR 975 elements with internal dimensions of a = 247.7 mm and b = 123.9 mm (width × height). The waveguide starts with the magnetron, followed by a circulator with a matched load, a directional coupler connected to a digital dual-channel microwave power meter, the MPS, and the movable plunger. The microwave generator and the circulator with matched load are connected by a common water cooling system. The movable plunger is element of the waveguide that enable minimisation of the reflected microwave power PR.
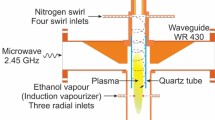
In this work, a metal-cylinder-based nozzleless MPS was employed to investigate hydrogen production from ethanol21,22. The MPS is based on the standard waveguide WR 975 with a length of λg (where λg represents the wavelength of a microwave frequency of 915 MHz in the standard waveguide WR 975, λg = 437 mm), featuring two additional metal cylinders on the wider side, as illustrated in Fig. 3. The MPS has a tapered section on the magnetron side. Starting from A–A′ plane, this section causes a linear change in the internal height of the waveguide from b to b1 = 31 mm over a length equivalent to λg/2. The height difference, b–b1, matches the height of the subsequently introduced flat middle section, which also has a length of λg/2. In the centre of the middle section, the metal cylinders are introduced containing a quartz tube in which the microwave plasma is generated in the form of flame. The flat middle section creates a reduced-height section inside the MPS which increases the intensity of the microwaves in the area where the plasma flame is generated. A carrier gas, which serves as the medium for initiating and sustaining the microwave plasma, is supplied to the MPS at atmospheric pressure. In this work nitrogen was used as the carrier gas. The presence of a quartz tube prevents the carrier gas from entering the interior of the waveguide. The carrier gas is introduced into the metal cylinder of the MPS above the waveguide through four inlets arranged tangentially to the circumference of the cross-section of this cylinder. This method of gas introduction creates a swirling flow inside the quartz tube, which provides protection against overheating and ensures stabilisation of the generated plasma21,22.
The ATW-06/500 inductive vapour generator (manufactured by ALGA) is capable of producing vapours of the liquid hydrogen carrier at temperatures up to 400 °C. The vapours are then supplied axially to the MPS, as shown in Fig. 3. The vapours are introduced into the plasma source through an inlet located in the axis of the metal cylinder above the MPS waveguide—the axial introducing hydrogen carrier vapour.
Figure 4 shows photos of plasma flames generated in nitrogen with and without the addition of ethanol vapour observed through the slit of the MPS. The images were taken with the volumetric flow rate of nitrogen QN2 = 2700 NL/h and the power PI = 5 kW. Ethanol vapour was added to the MPS at a rate of 0.8 kg/h and a temperature of 250 °C.
As illustrated in Fig. 4, the introduction of ethanol vapour resulted in an increase in the intensity of emitted radiation and a change in plasma colour. During the tests, a small amount of carbon deposits was observed on the inner wall of the quartz tube. Furthermore, the presence of ethanol vapour in the microwave plasma resulted in an increase in the observed length of the plasma flame. The experimental work with ethanol vapour in the microwave plasma allowed for the observation of general trends regarding the length of the generated plasma flame. It was found that an increase in the absorbed microwave power PA or a decrease in the volumetric flow rate of the carrier gas resulted in an increase in the length of the generated plasma flame.
Maximization the energy efficiency of the MPS
The cost of obtaining the discharge is a crucial factor in determining the applicability of the microwave plasma in the industry. To reduce the costs, it is essential to maximize the energy efficiency of the MPS. This can be done by identifying the minimum of the electrodynamic characteristics of the MPS. The electrodynamic characteristics refer to the relationship between the ratio PR/PI (where PI is the incident microwave power supplied to the MPS input plane) and the distance ls from the movable plunger to the MPS, see Fig. 3. The relationship PR/PI (ls) serves as a metric of the energy efficiency of the MPS, i.e. quantifying the effectiveness of absorbing microwave power by the generated plasma21,22. The absorbed microwave power PA by the generated plasma is defined as the difference between the power PI and PR. The minimum value in the relationship PR/PI (ls) indicates the optimal position of the movable plunger, ensuring the most efficient transfer of microwave energy from the microwave generator to the plasma in the MPS. The optimal position of the movable plunger can be determined through experimental measurements of the relationship PR/PI (ls).
The relationship PR/PI (ls) for microwave plasma in nitrogen was measured with and without the addition of the ethanol vapour, Fig. 5. The measurements were carried out under the following conditions: nitrogen with volumetric flow rate QN2 = 2700 NL/h, the power PI = 5 kW. Ethanol vapour was introduced into the MPS at a rate of 0.8 kg/h and a temperature of 250 °C.
In Fig. 5, the position of the movable plunger was normalised to the wavelength λg. The measured characteristics in nitrogen, with and without the addition of ethanol vapour, indicate the low energy efficiency of the MPS. The minimum of the PR/PI ratio for microwave plasma in nitrogen was greater than 0.25, and the range for stable discharge varied from 0.47 to 0.55. Upon introducing ethanol vapour, the PR/PI ratio increased and was higher than 0.4, also the range of normalised movable plunger positions decrease to range from 0.47 to 0.5. This suggests that the efficiency of microwave energy transfer to the plasma decreases after the introduction of the ethanol vapour and the stability of the generated discharge deteriorates. From a practical standpoint, this means that to sustain plasma in a mixture of nitrogen and ethanol, higher values of the power PI need to be supplied to the MPS compared to plasma in pure nitrogen to achieve the same value of the absorbed microwave power PA. It should also be noted that during the measurement the electrodynamic characteristics of the MPS at lower values of the power PI was challenging due to the unstable generation of microwave plasma. This instability prevented the acquisition of the data for lower values of the power PI than 5 kW.
Simulations of the distribution of the electric field module |E| in the MPS
Based on the measured electromagnetic characteristics of the MPS, it was concluded that the position ls/λg = 0.5 ensures the lowest value of the power PR (the minimum of the relationship PR/PI (ls/λg)). To confirm the maximization the energy efficiency of the MPS at ls/λg = 0.5, simulations of the distribution of the electric field module |E| inside the MPS were performed. The simulations were carried out to evaluate the effect of the position of the movable plunger and the presence of ethanol vapour on the distribution of the module |E| inside the MPS. The simulations were performed based on a two-port equivalent method proposed by Nowakowska et al.23, using a model of homogeneous microwave plasma flame24, i.e. the concentration of electrons at each point of the plasma column is the same. The transmission properties of such a two-port network can be described using the scattering matrix S. From simulations of the distribution of the electric field modulus |E| in the MPS, the elements of the scattering matrix S can be calculated. The RF module in the COMSL Multiphysics program was used for this purpose25.
In a chosen approach, a key factor is assumption of the appropriate shape of the generated plasma flame, an electron concentration ne and collision frequency ν, and a value of the relative electrical permittivity εp of the plasma. Based on the observations made during the measurement of the electrodynamic characteristic of the MPS, the generated plasma was assumed to have a column shape with a diameter d and height h. The adopted values of d and h for the considered cases of microwave plasma are listed in Table 1.
The plasma permittivity εp is assumed to be described by the Lorentz formula23,24:
where: n = ne/nc is the normalised electron concentration relative to the critical concentration nc, s = ν/ω is the normalized electron collision frequency relative to the angular frequency ω = 2πf, and a j = (− 1)1/2. The nc in the plasma is described by the equation23,24:
where ε0 is the electric permeability of vacuum (ε0 = 8.85 × 10−12 F/m), me is the mass of an electron (me = 9.1 × 10−31 kg), and e is the charge of an electron (e = 1.6 × 10−19 C).
Having established the dimensions of the plasma column and its relative electrical permittivity εp, the key parameters that determine the shape of the PR/PI (ls/λg) relationship are the ne and ν, the values of which are currently unknown. The ne and ν can be experimentally determined, for example by using optical emission spectroscopy (OES). In cases where experimental conditions do not allow to direct measurement, the values of the ne and ν can be estimated by numerically fitting the calculated relationships of the PR/PI (ls/λg) to the measured electrodynamic characteristics24.
Following the approach presented in Miotk et al.24 and a method of calculating the electrodynamic characteristics developed by Nowakowska et al.23, the calculated relationships PR/PI (ls/λg) were fitted to the measured experimental points by selecting appropriate values for n and s, as shown in Fig. 6. The least squares method was used as the fitting criterion for the calculated electrodynamic characteristics. The values of the ne and ν that gave the best fitting are as follows: in the case of nitrogen, the electron concentration and collision frequency were 2.55 × 1011 cm−3 and 5.75 × 108 s−1, respectively; whereas for nitrogen with ethanol vapour, the values were 2.39 × 1011 cm−3 and 3.16 × 108 s−1. The obtained values of the ne and ν showed the following trend: introducing ethanol vapour to the discharge area reduces the value of the electron concentration and collision frequency in the generated plasma.
By employing the applied plasma model and COMSOL Multiphysics software25, simulations of the distribution of the electric field module |E| in the MPS were performed. The objective of these simulations was to illustrate the impact of the movable plunger’s position on the distribution of the module |E|. The simulations were conducted for normalized positions of the movable plunger, noted as points (a), (b), (c), (d) and (e) in Fig. 6. The presented simulations were calculated in the case where the working gas was a mixture of nitrogen with added ethanol vapour. The position (d) represents the minimum of the electrodynamic characteristics of the MPS. On the other hand, position (e) corresponded to the maximum of the PR/PI (ls/λg) relationship. The results of the simulations are presented in Figs. 7 and 8. The electric field |E| module shown in these figures were normalized to a E0. The E0 represents the amplitude of the electric field intensity in a lossless and perfectly matched standard waveguide WR 975 (with inner wall conductivity σ = ∞), where only the H10 mode electromagnetic wave propagates. Scaling |E|/E0 enables the comparison of changes in the intensity of the module |E| within the MPS relative to the electric field intensity E0 in the waveguide transmission line. The amplitude E0 was determined using the relationship26:
where Zf is the impedance of the WR 975 waveguide with internal dimensions of a = 247.7 mm and b = 123.9 mm. The value of Zf can be determined using the following equation:
where: η ≈ 377 Ω is the impedance of free space, λ0 = 327.9 mm is the microwave wavelength at the frequency 915 MHz in free space. Using Eq. 3, for PI = 5 kW the amplitude E0 was equal to 18,107 V/m.
Simulated (top view) normalized electric field module |E|/E0 in the MPS for several movable plunger positions marked as (a)–(e) on Fig. 5. The simulations refer to the plane located at a height b1/2 of the reduced height waveguide.
Simulated (cross-section) normalized electric field module |E|/E0 in the MPS for several movable plunger positions marked as (a) and (b) on Fig. 5. The simulations refer to the plane located at a width a/2 of the reduced height waveguide.
The use of the |E|/E0 scale in Figs. 7 and 8 allowed to observation of a “focusing” effect of the electromagnetic field inside the MPS. This phenomenon is characterized by a significant increase of the |E|/E0 values around the plasma column at the movable plunger position (ls/λg = 0.5), which corresponds to the most efficient absorption of microwave power by the generated plasma (minimum PR/PI), Figs. 7d and 8d. At this position, the intensity of the module |E| is approximately ten times higher than E0, with |E| ≈ 10 × E0. On the other hand, Figs. 7e and 8e demonstrate simulations when the PR/PI ratio is close to one, indicating that all microwave power delivered to the input plane of the MPS was reflected. In this case the presented distributions show a complete disappearance of the electromagnetic field in the vicinity of the plasma column.
The simulation of the distribution of the module |E| in the MPS revealed that for the experimentally determined optimal movable plunger position, the simulated electromagnetic field reaches its peak in the area of microwave plasma generation, ensuring optimal conditions for plasma generation. Investigations into hydrogen production from ethanol vapour were carried out using this established movable plunger position, ls/λg = 0.5.
The analysis highlighted the need to improve the MPS in order to increase the transfer of microwave energy from the microwave source to the plasma. Increasing the transfer can be achieved by modifying the construction of the MPS. The modification of the construction of the MPS involves finding internal dimensions that ensure the lowest value of the ratio PR/PI in wide range of the lS. This can be done experimentally, which is expensive due to the physical search for the most optimal internal dimensions of the device or it can be done by first using simulations of the electric field module |E| in the MPS. The simulations enable to prediction of the impact of changes made inside the MPS on the relationships of the PR/PI (lS/λg). This method is more efficient and cost-effective in terms of time and finances compared to experimental approaches. Measurements of the electrodynamic characteristics and estimation of the ne and ν values are the first step in modifying the construction of the MPS using simulations. An example of such modifying can be found in Miotk et al.27.
Experimental results
This section presents the experimental results of hydrogen production from ethanol in microwave plasma. The investigations tested the following working conditions of the MPS: the power PA, the carrier gas volumetric flow rate, and the amount of the introduced ethanol vapours on the efficiency of hydrogen production. The aim was to achieve maximum efficiency in the hydrogen production, which was characterised by the following parameters:
-
production rate (g(H2)/h), the amount of hydrogen produced per unit of time;
-
percentage of hydrogen in the working gas after plasma treatment:
$$ [\left( {Q\left( {{\text{H}}_{{2}} } \right)_{{{\text{out}}}} /Q\left( {{\text{gases}}} \right)_{{{\text{out}}}} } \right] \times {1}00\% , $$
where Q(H2)out is the hydrogen flow rate at the plasma source outlet, and Q(gases)out is the total flow rate of working gas at the MPS outlet;
-
energy yield (g(H2)/kWh), an amount of hydrogen produced using 1 kWh of microwave energy absorbed by the generated plasma;where Q(EtOH)in represents the flow rate of the ethanol vapour introduced into the MPS, while Q(EtOH)out is the flow rate of the ethanol vapour at the plasma source outlet;
-
conversion rate of the ethanol (%): this refers to the proportion of the initial ethanol vapour that undergoes conversion in the plasma:
$$ \left[ {\left( {Q\left( {{\text{EtOH}}} \right)_{{{\text{in}}}} - Q\left( {{\text{EtOH}}} \right)_{{{\text{out}}}} } \right)/Q\left( {{\text{EtOH}}} \right)_{{{\text{in}}}} } \right] \times {1}00\% , $$where Q(EtOH)in represents the flow rate of the ethanol vapour introduced into the MPS, while Q(EtOH)out is the flow rate of the ethanol vapour at the plasma source outlet;
-
selectivity of the ethanol conversion to hydrogen (%): this indicates the proportion of the converted ethanol vapour that has been converted to hydrogen:
$$ \left[ {Q\left( {{\text{H}}_{{2}} } \right)_{{{\text{out}}}} /Q\left( {{\text{H}}_{{2}} } \right)_{{{\text{in}}}} } \right] \times {1}00\% , $$where Q(H2)out represents the flow rate of the hydrogen at the plasma source outlet, while Q(H2)in is the flow rate of the hydrogen in the ethanol vapour that introduced into the MPS.
Gas chromatography and IR spectrometry were used to determine the volumetric composition of the working gas after plasma treatment. Each gas sample was analysed at least three times. Thus, the results represent an average value. The precision of the working gas components concentrations was estimated by calculating standard deviation. Furthermore, due to the calibration procedure of the gas chromatography and IR spectrometry used error in gas components concentrations is finally estimated within a range of ± 5%.
This composition provides the necessary information to calculate the hydrogen production efficiency parameters.
The investigation of hydrogen production was carried out using processes of steam reforming of ethanol27,28,29:
-
steam reforming—is a process of converting hydrogen carriers into molecular hydrogen in the presence of water vapour, in the case of ethanol, this process may follows as:
$$ {\text{C}}_{{2}} {\text{H}}_{{5}} {\text{OH}} + {\text{3H}}_{{2}} {\text{O}} \to {\text{6H}}_{{2}} + {\text{2CO}}_{{2}} , $$(5)$$ {\text{C}}_{{2}} {\text{H}}_{{5}} {\text{OH}} + {\text{H}}_{{2}} {\text{O}} \to {\text{4H}}_{{2}} + {\text{2CO}}. $$(6)
The investigations of hydrogen production from ethanol vapour using steam reforming method showed a conversion rate of the ethanol over 99%, regardless of working conditions of the MPS. This means that the ethanol molecules were almost completely decomposed by the microwave plasma generated in our MPS.
Figures 9 and 10 shows the experimental results of hydrogen production by steam reforming of ethanol. In the tests the ratio of ethanol to water was 1:1 v/v liquid. Then, at a temperature of 250 °C, this mixture was introduced axially into the MPS in the form of vapours. The hydrogen production rate and energy yield were tested as a function of the absorbed microwave power PA by the generated plasma for two flow rates of nitrogen (the carrier gas): 2700 NL/h and 3900 NL/h, Fig. 9. Figure 10 shows the relationship between the hydrogen production rate, energy yield, and the amount of ethanol vapour introduced into the MPS. In our experiments, it proved impossible to maintain a stable microwave discharge at the ethanol flow rate above of 0.4 kg/h. This limitation restricts the potential for higher hydrogen production rates. This limitation may be due to an inappropriate ethanol/water ratio, which was 1:1 v/v in the test.
In Fig. 10, the hydrogen production rate and energy yield are strongly dependent on the amount of ethanol vapour introduced. These two important parameters of hydrogen production efficiency increased with the amount of introduced ethanol into the MPS. On the other hand, the increase in the absorbed microwave power PA by the generated plasma led to higher hydrogen production rates and a decrease in the energy yield of the obtained hydrogen, as shown in Fig. 9. However, above 4 kW of absorbed microwave power PA, hydrogen production decreases. This observed decrease in hydrogen production can be attributed to the high microwave power level, which increases the concentration of high-energy electrons. This results in more frequent ionisation and recombination events, altering reaction equilibria and favouring non-hydrogen pathways.
The water vapour added to the discharge, along with ethanol, served as a source of hydrogen in the plasma. As a result, the hydrogen produced in the plasma came from decomposition of the ethanol and water molecules. Therefore, the reported selectivity value refers to the mixture of ethanol and water, and ranges from 60.1% to 100%. The results show that the hydrogen production parameters increase with the amount of ethanol vapour introduced into the MPS. The highest hydrogen production parameters (production rate; energy yield) achieved in the steam reforming of ethanol method were 99.3 g(H2)/h and 26.9 g(H2)/kWh, as shown in Table 2. In addition, higher values of hydrogen production parameters were achieved for lower volumetric flow rates of nitrogen, as shown in Figs. 9 and 10.
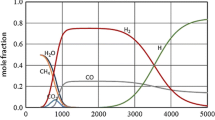
Figures 11 and 12 show the volumetric composition of the working gas after plasma treatment as a function of the absorbed microwave power PA and flow rate of ethanol vapour, respectively. The figures show that the main components of the working gas after plasma rotation are: nitrogen (up to 80% v/v), hydrogen (up to 25% v/v) and carbon monoxide (up to 8% v/v). Other components such as carbon dioxide, oxygen or acetylene (C2H2) are present in concentrations up to 2% v/v. In the tested range of working conditions of the MPS the amount of hydrogen in the working gas after plasma treatment varied from 6.3 to 25.7% v/v.
Table 3 summarises the impact of the working conditions of the MPS on the efficiency of hydrogen production parameters. The investigations showed that the increase in the absorbed microwave power PA by the generated plasma resulted in higher hydrogen production rate, selectivity, and volumetric percentage of hydrogen in the working gas after plasma treatment. This phenomenon is explained as follows: an increase in the absorbed microwave power PA lengthens the plasma column, resulting in a longer residence time of the reactants in the discharge region. Furthermore, increasing the amount of ethanol vapour introduced into the MPS resulted in an increase in all parameters characterising the efficiency of hydrogen production except selectivity. Moreover, an increase in the flow rate of nitrogen led to a decrease in all efficiency parameters that characterise hydrogen production efficiency.
The efficiency of hydrogen production is significantly influenced by the flow rate of ethanol vapour and the absorbed microwave power PA. Higher flow rate of ethanol vapour facilitate hydrogen production by increasing the availability of reactants. However, excessive levels can result in plasma saturation and instability. Conversely, higher absorbed microwave power PA values boost reaction rates by generating more high-energy electrons, but excessive power can lead to overheating and reduced efficiency. A balance is therefore required: the ethanol vapour concentration should be high enough to ensure an adequate supply of reactants, but not so high as to cause saturation or instability. Concurrently, the absorbed microwave power PA must be sufficient to sustain the energetic plasma without causing overheating.
The DOE requires a minimum energy yield of 60 g(H2)/kWh for hydrogen production technology to be accepted by the industry, whether in distributed or centralized systems. Table 4 compares the energy yield of hydrogen production from liquid hydrogen carriers using plasma methods. The term ‘catalyst’ in parentheses in the ‘Production method’ column indicates the use of a catalyst in the method, which increases the efficiency of hydrogen production. However, opinions are divided on the potential of catalysts in commercial applications to support hydrogen production using plasma methods1. The high cost of the catalyst and its susceptibility to contamination can reduce its effectiveness and contribute to the overall operating costs of the process.
The Table 4 shows that literature analysis has mainly focused on plasma conversion of liquid substances for hydrogen production from ethanol and methanol. The plasma technologies are not listed in the DOE report, although some are currently approaching the 60 g(H2)/kWh requirement. These are glow discharge, spark discharges, and microwave discharges. The methods developed by Yan et al.31, Xin et al.38, Ulejczyk et al.15, Zhu et al.42 and Sun et al.44 are the closest to meeting the DOE requirements.
The energy yield obtained in this work is below the DOE criterion of 60 g(H2)/kWh. It is important to note that the industry is constantly evolving and changing, and the DOE recommendation is not the only factor that determines the acceptance of hydrogen production methods. With the evolution of the industry, additional criteria have emerged, including resource extraction, production, processing, transportation, utilization, retail sales, and waste disposal. These factors can significantly impact the final implementation of a method. Therefore, the energy efficiency of hydrogen production for different technologies is often not the sole parameter determining the competitiveness of a method, and other criteria must be considered. A method with lower hydrogen production efficiency parameters may still be competitive and worth implementing when other criteria outweigh it.
Summary
Experimental measurement of the electrodynamic characteristics and simulations of the |E| field distribution enabled the determination of the movable plunger position that provided the most efficient energy transfer to the generated plasma in the MPS. The position was ls/λg = 0.5. The adopted microwave plasma model enabled the estimation of electron concentration ne and collision frequency ν in the generated plasma. The conducted studies indicated that the introduction ethanol vapour into the discharge arena led to a decrease in ne and ν values. Furthermore, analysing the electrodynamic characteristics indicated the need to modify the construction of the MPS. The aim of these modifications is to improve the efficiency of the transfer of microwave energy from the microwave source to the plasma and to expand the range for stable discharge in the MPS.
The main aim of the presented investigations was to experimentally test the impact of the selected working conditions of the MPS on the efficiency of hydrogen production from steam reforming of ethanol. The highest energy yield of producing hydrogen achieved the rate of 26.9 g(H2)/kWh, while the highest hydrogen production rate was 99.3 g(H2)/h. It should be noted that the process of steam reforming of ethanol could only be achieved within a limited range of ethanol vapours introduced into the MPS. Stable microwave discharge could not be sustained above 0.4 kg/h, limiting the potential for higher hydrogen production rates. This limitation may result from an improper ratio of ethanol to water, which was tested at 1:1 v/v. Water vapour is a strong microwave absorber and is likely to absorb a significant proportion of the microwave energy transferred to the MPS, leading to instability in the microwave discharge produced. It is worth noting that the theoretical limit of energy yield for hydrogen production in the steam reforming of ethanol vapour is 249 g(H2)/kWh, Eq. (5). With a higher amount of ethanol vapour introduced, the steam reforming process is expected to be more efficient than in the present work, which will be the aim of our next investigations.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Mizeraczyk, J., Urashima, K., Jasiński, M. & Dors, M. Hydrogen production from gaseous fuels by plasmas—a review. Int. J. Plasma Environ. Sci. Technol. 8(2), 89–97 (2014).
Ahmad, T. & Zhang, D. A critical review of comparative global historical energy consumption and future demand: The story told so far. Energy Rep. 6, 1973–1991. https://doi.org/10.1016/j.egyr.2020.07.020 (2020).
U. S. Department of Commerce, National Oceanic and Atmospheric Administration Earth System Research Laboratory, Global Monitoring Division: http://www.esrl.noaa.gov/gmd/ccgg/trends/full.html.
Audi, M., Ali, A. & Kassem, M. Green house gases: A review of losses and benefits, Munich Personal RePEc Archive Paper No. 98108 (2020).
Buckley, N. A., Juurlink, D. N., Isbister, G., Bennett, H. M. & Lavonas, E. J. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst. Rev. 4, CH002041 (2011).
Singh, A. A., Eram, R., Agrawal, M. & Agrawal, S. B. Air pollution: sources and its effects on humans and plants. Int. J. Plant Environ. 8(1), 10–24. https://doi.org/10.18811/ijpen.v8i01.02 (2022).
Khasanova, S., Alieva, E. & Shemilkhanova, A. Environmental pollution: types, causes and consequences. BIO Web of Conferences 63, 07014. https://doi.org/10.1051/bioconf/20236307014 (2023).
Auffant, M. Oil for atoms: The 1970s energy crisis and nuclear proliferation in the Persian Gulf. Texas Natl. Secur. Rev. 5(3), 60–82. https://doi.org/10.26153/tsw/42079 (2022).
U. S. Department of Energy, Hydrogen and fuel cells program. In 2013 Annual Merit Review and Peer Evaluation Report (2013).
Gahleitner, G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrogen Energy 38, 2039–2061. https://doi.org/10.1016/j.ijhydene.2012.12.010 (2013).
D. Papageorgopoulos, Fuel cell technologies overview. In 2022 Annual Merit Review and Peer Evaluation Meeting (2022).
Malik, F. R., Yuan, H., Moran, J. C. & Tippayawong, N. Overview of hydrogen production technologies for fuel cell utilization. Eng. Sci. Technol. 43, 101452. https://doi.org/10.1016/j.jestch.2023.101452 (2023).
K. Randolph, U. S. DOE, Hydrogen production. In 2013 Annual Merit Review and Peer Evaluation Meeting (2013).
U.S. DRIVE, Hydrogen Production Tech Team Roadmap (2017).
Ulejczyk, B., Nogal, Ł, Młotek, M. & Krawczyk, K. Efficient plasma technology for the production of green hydrogen from ethanol and water. Energies 15, 2777. https://doi.org/10.3390/en15082777 (2022).
Budhraja, N., Pal, A. & Mishra, R. S. Plasma reforming for hydrogen production: Pathways, reactors and storage. Int. J. Hydrogen Energy 48(7), 2467–2482. https://doi.org/10.1016/j.ijhydene.2022.10.143 (2023).
Pacheco, J. et al. Greenhouse gas treatment and H2 production, by warm plasma reforming. Int. J. Hydrogen Energy 40, 17165–17171. https://doi.org/10.1016/j.ijhydene.2015.08.062 (2015).
Chen, F., Huang, X., Cheng, D. & Zhan, X. Hydrogen production from alcohols and ethers via cold plasma: A review. Int. J. Hydrogen Energy 39, 9036–9046. https://doi.org/10.1016/j.ijhydene.2014.03.194 (2014).
Akande, O. & Lee, B. Plasma steam methane reforming (PSMR) using a microwave torch for commercial-scale distributed hydrogen production. Int. J. Hydrogen Energy 47, 2874–2884. https://doi.org/10.1016/j.ijhydene.2021.10.258 (2022).
Rincon, R., Munoz, J., Morales-Calero, F. J., Orejas, J. & Calzada, M. D. Assessment of two atmospheric-pressure microwave plasma sources for H2 production from ethanol decomposition. Appl. Energy 294(11), 116948. https://doi.org/10.1016/j.apenergy.2021.116948 (2021).
Mizeraczyk, J., Jasiński, M., Nowakowska, H. & Dors, M. Studies of atmospheric-pressure microwave plasmas used for gas processing. Nukleonika 57(2), 241–247 (2012).
Miotk, R. et al. Liquid fuel reforming using microwave plasma at atmospheric pressure. Plasma Sources Sci. Technol. 25, 035022. https://doi.org/10.1088/0963-0252/25/3/035022 (2016).
Nowakowska, H., Jasiński, M. & Mizeraczyk, J. Electromagnetic field distributions in waveguide-based axial-type microwave plasma source. Eur. Phys. J. D 54, 511–518. https://doi.org/10.1140/epjd/e2008-00259-y (2009).
Miotk, R., Jasiński, M. & Mizeraczyk, J. Analysis of the tuning characteristics of microwave plasma source. Phys. Plasmas 23(4), 3507–3510. https://doi.org/10.1063/1.4946879 (2016).
Panecki, M., Litwin, R. & Drozdowicz, L. Teoria i Technika Mikrofalowa (WNT, 1972).
Miotk, R., Jasiński, M. & Mizeraczyk, J. Improvement of energy transfer in a cavity-type 915-MHz microwave plasma source. IEEE Trans. Microw. Theory Tech. 66(2), 711–716. https://doi.org/10.1109/TMTT.2017.2778068 (2018).
Petitpas, G. et al. A comparative study of non-thermal plasma assisted reforming technologies. Int. J. Hydrogen Energy 32, 2848–2867. https://doi.org/10.1016/j.ijhydene.2007.03.026 (2007).
Sun, S., Yan, W., Sun, P. & Chen, J. Thermodynamic analysis of ethanol reforming for hydrogen production. Energy 44, 911–924. https://doi.org/10.1016/j.energy.2012.04.059 (2012).
Liu, X. Z., Liu, C. & Eliasson, B. Hydrogen production from methanol using corona discharges. Chin. Chem. Lett. 14, 631–633 (2003).
Yan, X. C., Li, C. & Lin, W. H. Hydrogen generation by glow discharge plasma electrolysis of methanol solutions. Int. J. Hydr. Energ. 34, 48–55. https://doi.org/10.1016/j.ijhydene.2008.09.099 (2009).
Meiyazhagan, S., Yugeswaran, S., Suresh, K., Ananthapadmanabhan, P. V. & Kobayashi, A. Feasible production of hydrogen from methanol reforming through single stage DC microplasma reactor. Int. J. Mod. Phys. B 34, 2050108. https://doi.org/10.1142/S0217979220501088 (2020).
Burlica, R., Shih, K. & Locke, B. R. Formation of H2 and H2O2 in a water-spray gliding arc nonthermal plasma reactor. Ind. Eng. Chem. Res. 49, 6342–6349. https://doi.org/10.1021/ie100038g (2010).
Zhang, H., Zhu, F., Bo, Z., Cen, K. & Li, X. Hydrogen production from methanol decomposition in a gliding arc discharge plasma with high processing capacity. Chem. Lett. 44, 1315–1317. https://doi.org/10.1246/cl.150563 (2015).
Zhang, H. et al. Enhanced hydrogen production by methanol decomposition using a novel rotating gliding arc discharge plasma. RSC Adv. 6, 12770. https://doi.org/10.1039/C5RA26343C (2016).
Pang, Y., Bosch, H., Hammer, T., Müller, D. & Karl, J. Plasma-aided reforming of toluene and isopropanol with analysis of decomposition mechanism. Waste Biomass Valor. 11, 675–688. https://doi.org/10.1007/s12649-019-00648-3 (2020).
Malik, M. A., Hughes, D., Malik, A., Xiao, S. & Schoenbach, K. H. Study of the production of hydrogen and light hydrocarbons by spark discharges in diesel, kerosene, gasoline, and methane. Plasma Chem. Plasma Process. 33, 271–279. https://doi.org/10.1007/s11090-012-9429-1 (2013).
Xin, Y. et al. Characteristics of hydrogen produced by pulsed discharge in ethanol solution. Appl. Energy 168, 122–129. https://doi.org/10.1016/j.apenergy.2016.01.092 (2016).
Ulejczyk, B., Nogal, Ł, Młotek, M. & Krawczyk, K. Enhanced production of hydrogen from methanol using spark discharge generated in a small portable reactor. Energy Rep. 8, 183–191. https://doi.org/10.1016/j.egyr.2021.11.290 (2022).
Hrycak, B. et al. Hydrogen production from ethanol in nitrogen microwave plasma at atmospheric pressure. Open Chem. 13, 317–324. https://doi.org/10.1515/chem-2015-0039 (2015).
Tsyganov, D., Bundaleska, N., Tatarova, E. & Ferreira, C. Ethanol reforming into hydrogen-rich gas applying microwave ‘Tornado’-type plasma. Int. J. Hydrogen Energy 38, 14512–14530. https://doi.org/10.1016/j.ijhydene.2013.08.127 (2013).
Zhu, T., Sun, B., Zhu, X., Wang, L. & Xin, Y. Mechanism analysis of hydrogen production by microwave discharge in ethanol liquid. Int. J. Hydrogen Energy 156, 105111. https://doi.org/10.1016/j.jaap.2021.105111 (2021).
Wang, B. et al. Hydrogen production from alcohol solution by microwave discharge in liquid. Int. J. Hydrogen Energy 41, 7280–7291. https://doi.org/10.1016/j.ijhydene.2016.03.110 (2016).
Sun, B., Zhao, X., Xin, Y. & Zhu, X. Large capacity hydrogen production by microwave discharge plasma in liquid fuels ethanol. Int. J. Hydrogen Energy 42, 24047–24054. https://doi.org/10.1016/j.ijhydene.2017.08.052 (2017).
Czylkowski, D., Hrycak, B., Jasiński, M., Dors, M. & Mizeraczyk, J. Hydrogen production by direct injection of ethanol microdroplets into nitrogen microwave plasma flame. Int. J. Hydrogen Energy 43, 21196–21208. https://doi.org/10.1016/j.ijhydene.2018.09.143 (2018).
Guo, W., Zheng, X., Qin, Z., Guo, Q. & Liu, L. Hydrogen production from ethanol reforming by a microwave discharge using air as a working gas. ACE Omega 6, 33533–33541. https://doi.org/10.1021/acsomega.1c04312 (2021).
Niu, Y. & Li, S. Hydrogen production by decomposition of ethanol in the afterglow of atmospheric-pressure nitrogen microwave plasma torch. J. Phys. D Appl. Phys. 56, 085203. https://doi.org/10.1088/1361-6463/acb448 (2023).
Batukaev, T., Bilera, I., Krashevskaya, G., Lebedev, Y. & Epstein, I. Hydrogen production in microwave discharge in water solutions of ethanol at atmospheric pressure. Plasma Process Polym. 20, 2300015. https://doi.org/10.1002/ppap.202300015 (2023).
Acknowledgements
We are grateful to the Institute of Fluid Flow Machinery PAS for the support of this work.
Author information
Authors and Affiliations
Contributions
All the authors made substantial contributions to the experimental investigations as well as to the preparing of the submitted manuscript. All of them have approved the submitted version of the manuscript. The particular contribution of the authors was as follows: R.M.—corresponding author, performing simulations, preparation of the manuscript tables and figures, writing the main text of the manuscript. B.H. and D.C.—preparation of the experimental setup, conduction of the plasma experiments, analysis and interpretation of the experimental data. M.J.—the idea of the geometry of the presented MPS, conceptualization and methodology of the research, supervision all the work. M.D.—the idea of the geometry of the presented MPS, funding acquisition, conceptualization and methodology of the research. J.M.—funding acquisition, project administration, conceptualization and methodology of the research, interpretation of the experimental data, preparation of the manuscript tables, writing the main text of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miotk, R., Hrycak, B., Czylkowski, D. et al. Atmospheric pressure microwave (915 MHz) plasma for hydrogen production from steam reforming of ethanol. Sci Rep 14, 14959 (2024). https://doi.org/10.1038/s41598-024-65874-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65874-9
- Springer Nature Limited