Abstract
To elucidate the currently unknown relationship between hyperthyroidism and osteoarthritis (OA). During 2007–2012, 7,433 participants (hyperthyroidism patients = 125; OA patients = 675) were included in the National Health and Nutrition Examination Survey database. We used a weighted multivariable-adjusted logistic regression analysis to assess the association between hyperthyroidism and OA. We also assessed the causality of that relationship using publicly available genome-wide association study data and three Mendelian randomization (MR) analysis methods. The heterogeneity test, pleiotropy test, and leave-one-out tests were used for sensitivity analysis. In this cross-sectional study, after adjusting for potential confounding factors, we found that hyperthyroidism significantly (P = 0.018) increased the risk of OA (odds ratio [OR] = 2.23, 95% confidence interval [CI] = 1.2–4.17). Age-stratified analysis revealed that hyperthyroidism was associated with a greater risk of OA in the 60–80-year-old age group (OR = 2.86, 95% CI = 1.46–5.59, P = 0.002), with no significant association in the 18–59-year-old age group (all P > 0.05). The results of the inverse-variance weighting (IVW) analysis showed that hyperthyroidism increased the risk of OA (OR = 1.23, 95% CI = 1.04–1.46; P = 0.017). The weighted median estimator (WME) and MR-Egger method also confirmed this causal association (OR = 1.27 and OR = 1.32, respectively). The sensitivity analysis results confirmed the reliability of this conclusion. In addition, IVW-based reverse-MR analysis revealed that OA did not increase the risk of hyperthyroidism (OR = 1.02, 95% CI = 0.97–1.08; P = 0.449). Hyperthyroidism is associated with an increased risk of OA, but the underlying pathological mechanism still needs to be clarified in future research.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a degenerative bone and joint disease caused by articular cartilage injury, hyperosteogeny, subchondral osteosclerosis, and pathological changes in the synovium and/or articular cavity1,2 and is mainly characterized by joint pain and dysfunction. The pathogenesis of OA is relatively complex and is related to factors such as inflammation, loss of joint fluid, cytokine levels, and metabolic abnormalities3,4. OA typically occurs among middle-aged and elderly people and has a high incidence, disability rate, and mortality rate, imposing a serious economic burden on patients' families and society2,5. A study based on the global burden of disease data showed that6 compared with that for lower back and neck pain, the age-standardized incidence rate (ASIR) for OA increased by 0.32% (95% CI 0.28–0.36) annually worldwide or approximately 9% over 28 years (from 1990 to 2017). The recognized causes of OA include obesity, genetic factors, ageing, inflammation, and trauma3,4,7, and joint cartilage degeneration, caused by the combined effects of these factors, is the core link in the pathogenesis of OA. In addition, some disease-related factors, such as fatigue and infection events, have also been found to be associated with OA8,9,10. Heijman et al. reported that OA patients experience a stable daily level of fatigue9, which means that OA patients have a worse quality of life than healthy people. Patients with end-stage OA may inevitably need surgical treatment10, and possible infection events may not only increase the health and economic burden but also seriously reduce quality of life. Undoubtedly, early identification of controllable factors that increase the risk of OA and management of these factors have important clinical and public health value in reducing the incidence of OA or delaying its progression.
Thyroid dysfunction is believed to be associated with OA11,12, but strong evidence supporting this potential correlation is lacking. Krieger et al.'s research showed that thyroid-stimulating hormone (TSH) and other thyroid hormones (THs) can induce the occurrence of OA13. Numerous studies have shown that TSH can act on target organs outside the thyroid gland (such as articular cartilage)14,15,16 and is involved in the occurrence and development of various diseases. Hyperthyroidism is a common disease of the endocrine system caused by an increase in the synthesis or secretion of THs17. A clinical study involving 109 patients with thyroid dysfunction revealed a significant association between hyperthyroidism and knee joint pain; the included patients had abnormal muscle–bone ultrasound findings12. However, there is still a lack of evidence supporting the association between hyperthyroidism and OA. To fill this gap, we utilized data from the National Health and Nutrition Examination Survey (NHANES) database to investigate the relationship between hyperthyroidism and OA, which was our primary research goal. For our second research goal, we used a two-sample Mendelian randomization (MR) method to explore whether there was a causal relationship between hyperthyroidism and OA. Our hypothesis was that there is a positive causal relationship between hyperthyroidism and OA and that there is no reverse causal relationship. The conclusions of this study can provide a reliable basis for preventing or delaying the progression of OA in hyperthyroidism patients.
Materials and methods
Cross-sectional study
Data sources and study participants
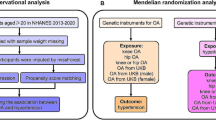
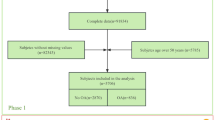
We used NHANES data from 2007 to 2008, 2009 to 2010, and 2011 to 2012; laboratory data on thyroid function were reported during these three survey cycles. The NHANES is a cross-sectional survey conducted by the Centers for Disease Control and Prevention (CDC) in the United States18. All participants signed an informed consent form19, and the use of NHANES data was approved by the Ethics Review Committee of the National Center for Health Statistics in the United States19. Thus, no external ethical approval or informed consent was needed. For the 2007–2012 survey period, a total of 10,600 participants had complete thyroid function measurement data. To accurately study the relationship between hyperthyroidism and OA, we excluded participants with rheumatoid arthritis, other types of arthritis, psoriatic arthritis, or hypothyroidism. In addition, all participants lacking OA status and thyroid function information were not included for further analysis, and 7433 participants were ultimately included. The inclusion and exclusion process for the research participants is shown in Fig. 1.
Determination of OA outcomes
The diagnosis of OA was mainly determined from the questionnaire survey results. In the NHANES questionnaire, the participants were asked "Has a doctor or other health professional ever told you that you have arthritis?". If a participant answered “yes”, the question "Which type of arthritis do you have?" was subsequently asked. Participants who self-reported having osteoarthritis were considered OA patients for the purpose of the present study. Previous studies have confirmed the effectiveness of self-reported OA diagnoses20,21.
Measurement of thyroid function
The diagnosis of hyperthyroidism was based on the participants’ reported drug use and TSH test results, and this evaluation method was similar to that used by Kakigi et al. and Airaksinen et al.22,23. We collected TSH data measured by the NHANES from 2007 to 2012, as well as data on drug usage. To evaluate thyroid function, blood samples were collected from all participants at the NHANES Mobile Examination Center (MEC) and transported to the collaborating laboratory service centre in Ottumwa, Iowa24. TSH measurements were quantitatively analysed using the third-generation dual-site immune enzyme high-sensitivity human TSH method24. According to the reagent manufacturer guidelines, the normal TSH range is 0.34–5.6 mIU/mL25. Participants who had not received TH replacement treatment or antithyroid drug treatment and whose TSH value was 0.34–5.6 mIU/mL were classified as having normal thyroid function26,27. Participants who self-reported current levothyroxine use, had a desiccated thyroid (regardless of their TSH level) or whose TSH level was greater than 5.6 mIU/mL and who did not take antithyroid drugs were classified as having hypothyroidism26,27. Participants who reported current methimazole or propylthiouracil use (regardless of their TSH level) or who had a TSH level less than 0.34 mIU/mL were classified as having hyperthyroidism26,27.
Assessment of covariates
The covariates included in this cross-sectional study were mainly sociodemographic factors and included age, sex, race, body mass index (BMI), smoking status, and alcohol consumption. We divided the patients into three groups based on BMI values: < 25.0, 25.0–29.9, and ≥ 30 kg/m2. According to Rattan et al.'s classification criteria28, we classified alcohol consumption levels into never, former, low, moderate, and heavy. In addition, we included six diseases, diabetes29, chronic kidney disease (CKD)30, hypertension31, hyperlipidaemia32, chronic obstructive pulmonary disease (COPD)33, and coronary heart disease (CHD)34, as covariates. The diagnoses for these six diseases were based on clear diagnostic criteria29,30,31,32,33,34 and matched with measurement indicators in the NHANES database; the participants included in this study voluntarily reported taking disease-related drugs, such as hypoglycaemic drugs and antihypertensive drugs, or reported having been diagnosed by a doctor. In addition, to further explore the association between hyperthyroidism status and the risk of developing OA in different age groups, subgroup analysis was also performed in this study.
MR study
Assumptions and data sources for the MR analysis
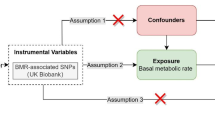
MR analysis is considered to meet the three core assumptions of relevance, independence, and exclusivity, which are the foundation of this type of analysis. In the MR analysis in this study, the three specific assumptions are summarized as follows: (1) the instrumental variables (IVs) had to be strongly related to the exposure factors (hyperthyroidism); (2) the IVs could not be associated with any confounding factors associated with the "exposure-outcome"; and (3) the IVs could only affect the outcome variable (OA) through exposure factors (hyperthyroidism) and could not be affected by other factors. The hypothesis for the MR analysis in this study is shown in Fig. 2. The genome-wide association study (GWAS) target population consisted entirely of Europeans, and both males and females were included in this study. The data source was the Integrative Epidemiology Unit (IEU) analysis of UK Biobank phenotypes (https://gwas.mrcieu.ac.uk/). The GWAS included data from 337,159 hyperthyroidism patients, with 2547 included in the case group and 334,612 included in the control group (ID: ukb-a-76). A total of 10,894,596 single nucleotide polymorphisms (SNPs) were detected. The sample size in the OA GWAS data was 462,933 participants, including 38,472 OA patients and 424,461 non-OA patients (ID: ukb-b-14486), with a total of 985,1867 SNPs. The specific information for the data sources is provided in Supplementary Material 1.
Genetic variants associated with hyperthyroidism
The selection of IVs satisfied the first assumption of the MR hypothesis in this study; that is, IVs (SNPs) had to have a strong correlation with hyperthyroidism. With P < 5 × 10−8 as the screening criterion, SNPs that were strongly correlated with hyperthyroidism were screened from the GWAS data. On this basis, we set the parameters (distance > 10 MB and r2 < 0.001) to run the data filtering program to conduct linkage disequilibrium pruning of the SNPs. If there was no strong correlation between the genetic variation and the exposure factors or if the genetic variation could only explain a small part of the phenotypic variation, the genetic variation was referred to as weak35. We evaluated whether the IVs selected were weak by calculating F statistics35.
Second, to ensure that the second MR hypothesis was met, we queried all the IVs that met the assumption of the first hypothesis in the PhenoScanner database (http://www.phenoscanner.medschl.cam.ac.uk/) to ensure that these IVs were not associated with known confounding factors (BMI, age, oestrogen reduction) (Supplementary Material 2).
Finally, we extracted the SNPs selected in the steps described above from the GWAS data of the outcome variable (OA). In addition, SNPs related to OA were excluded (P < 5 × 10−8).
Statistical analysis
Cross-sectional analysis
The data analysis strategy in this section was conducted in accordance with the CDC's statistical guidelines36. All data analyses were conducted using R software (version 4.2.2, http://www.R-project.org, The R Foundation) and EmpowerStats software (version 4.1, www.empowerstats.com, X&Y solutions, Inc. Boston, MA, USA). All tests were bilateral and had a significance level of α = 0.05. Categorical variables are expressed as frequencies and weighted percentages. We used a binary logistic regression model to evaluate the relationship between hyperthyroidism and OA. To test the stability of the relationship between hyperthyroidism and OA, we constructed three regression models to gradually adjust for confounding factors: Model 1 was not adjusted for covariates; Model 2 was adjusted for age, sex, BMI, and race; and Model 3 expanded on Model 2 and was further adjusted for smoking status, alcohol consumption, diabetes status, CKD status, hypertension status, hyperlipidaemia status, COPD status, and CHD status.
MR analysis
MR analysis was performed using the TwoSampleMR package in R software (version 4.2.2, http://www.R-project.org, The R Foundation). We used inverse variance weighting (IVW) random effects, the weighted median estimator (WME) and MR‒Egger for MR analysis and evaluated the potential causal relationship between hyperthyroidism and OA with odds ratios (ORs) and 95% confidence intervals (CIs). The principle of IVW is to calculate the weighted average of all IVs' effect sizes on the premise of ensuring that all IVs are valid and not considering intercept terms in regression analysis37, which is considered the main statistical method of MR analysis.
To evaluate whether OA had a causal effect on hyperthyroidism, we conducted reverse MR analysis with OA as the exposure and hyperthyroidism as the outcome. The process for selecting IVs and for MR analysis is described above. The final IV information for the reverse MR analysis can be found in Supplementary Material 3.
To further test the validity and robustness of the statistical results, we conducted sensitivity analyses. In this MR analysis, three methods, namely, the heterogeneity test, pleiotropy test, and leave-one-out (LOO) sensitivity test, were used for sensitivity analyses38,39.
Ethics approval and consent to participate
The protocols of NHANES were approved by the institutional review board of the National Center for Health Statistics, CDC (https://www.cdc.gov/nchs/nhanes/irba98.htm). NHANES has obtained written informed consent from all participants.
Results
Cross-sectional study
Baseline characteristics of the study participants
A total of 7,433 participants (18–80 years old) were included in this study, with 675 OA patients identified from personal interview data, including their self-reported health status; the remaining 6758 participants were all non-OA patients. In terms of thyroid function, a total of 125 participants (1.4%) were identified as having hyperthyroidism, while the other 7308 participants (98.6%) had normal thyroid function. The weighted percentages of hyperthyroidism incidence in OA and non-OA participants were 3.0% and 1.3%, respectively, and the difference between the two groups was statistically significant (P = 0.008). All covariates included in this cross-sectional study showed statistically significant differences between OA patients and non-OA patients (P < 0.05). The basic characteristics of the included participants based on OA status are shown in Table 1.
Association between hyperthyroidism and OA
We constructed three logistic regression models to explore the independent impact of hyperthyroidism on OA (Table 2). The unadjusted model (Model 1) results showed that hyperthyroidism significantly increased the risk of OA (OR = 2.4, 95% CI 1.23–4.71; P = 0.014). The results of the microadjustment model (Model 2) and the model adjusted for all covariates (Model 3) showed that hyperthyroidism increased the risk of OA, and the corresponding effect sizes (and 95% CIs) were OR = 2.11 (95% CI 1.15–3.87, P = 0.02) and OR = 2.23 (95% CI 1.2–4.17, P = 0.018), respectively. The results of the logistic regression analysis indicated a positive correlation between hyperthyroidism and the risk of OA.
Considering the close relationship between the OA status and age of the surveyed population, we analysed the association between hyperthyroidism status and OA risk in different age groups (18–34, 35–49, 50–59, and 60–80 years). The statistical results indicated that there was no significant correlation between hyperthyroidism status and the risk of OA in the populations aged 18–34 (P = 0.992), 35–49 (P = 0.783), or 50–59 (P = 0.452) years. In the population aged 60–80 years, hyperthyroidism was positively correlated with a greater risk of OA (OR = 2.86, 95% CI 1.46–5.59, P = 0.002). All these statistical results can be found in Table 2.
MR study
Bidirectional MR analyses of hyperthyroidism and OA
To meet the three major assumptions of this MR study, a total of 6 SNPs were used for MR analysis (Table 3). MR analysis based on the IVW method revealed a causal relationship between hyperthyroidism and OA, indicating that hyperthyroidism increases the risk of OA (OR = 1.23, 95% CI 1.04–1.46; P = 0.017) (Fig. 3A). In addition, MR analysis based on the WME and MR-Egger methods also revealed that hyperthyroidism increases the risk of OA, and the statistical results obtained with these two methods were similar to those obtained with the IVW method (OR = 1.27 and OR = 1.32, respectively).
To confirm whether there is a reverse causal relationship between hyperthyroidism and OA, we conducted reverse MR analysis (Fig. 3B). The IVW results showed that OA did not increase the risk of hyperthyroidism (OR = 1.02, 95% CI 0.97–1.08, P = 0.449), and this conclusion was also confirmed by the statistical results obtained with the WME (OR = 1.02, 95% CI 0.96–1.08, P = 0.572) and MR‒Egger methods (OR = 0.93, 95% CI 0.84–1.04, P = 0.265).
Sensitivity analysis
The heterogeneity test results showed that the Q and P for the IVW and MR-Egger Q values were 4.91 (0.426) and 4.71 (0.318), respectively, indicating that heterogeneity did not exist. We used the MR-Egger regression intercept to verify the existence of pleiotropy in this study. The statistical results showed that the Egger intercept value was − 0.0002 (close to 0), and P = 0.699, indicating that there was no horizontal pleiotropy in this study. The LOO results showed that the causal relationship between hyperthyroidism and an increased risk of OA did not change after systematically removing a single SNP and repeating IVW-based MR analysis (Fig. 4).
Discussion
There is a lack of strong research reporting on the association between hyperthyroidism and OA, and our study fills this gap. We explored the relationship between hyperthyroidism and OA based on cross-sectional NHANES and GWAS data and aimed to verify the potential causal relationship between hyperthyroidism and OA to provide convincing evidence. The results of this cross-sectional study indicate a positive linear relationship between hyperthyroidism and OA, indicating that hyperthyroidism increases the risk of OA in the population aged 60–80 years. In addition, two-sample MR analysis revealed that hyperthyroidism increases the risk of OA via a significant causal relationship, and this conclusion was not affected by a reverse causal relationship; that is, OA does not increase the risk of hyperthyroidism. The findings of this study will provide strong guidance for the prevention and treatment of OA, which means that identifying relevant targets for hyperthyroidism treatment may reveal new ideas for the pharmaceutical treatment of OA. In addition, the conclusions of this study also provide a basis for the public health system to strengthen the monitoring of OA occurrence in hyperthyroidism patients.
Cartilage injury, subchondral bone destruction, and chondrocyte hypertrophy are significant pathological features of OA40,41 and are also the main pathological factors for the occurrence and progression of OA. Rim et al. showed that the TSH receptor is highly expressed in chondrocytes42, but the role and mechanism of TSH in chondrocyte differentiation have not yet been revealed. One study showed that osteoporosis can affect overall bone mass43, and abnormalities in the microstructure of subchondral bone tissue may lead to uneven stress on articular cartilage, which may lead to secondary cartilage damage and osteophyte hyperplasia, thereby promoting the occurrence and progression of OA. Tokgoz et al. reported that greater bone mineral density (BMD) can delay the progression of knee osteoarthritis (KOA)44. The impact of thyroid-related hormone levels on bone tissue has long been studied by scholars45,46, and among these impacts, TH and TSH have positive regulatory and maintenance effects on bone development47,48. High TH levels in hyperthyroidism patients lead to an increase in the bone turnover rate and an imbalance in the proportion of bone resorption and bone formation processes49. Moreover, the inhibition of TSH can also affect the bone regulatory effect of TSH49,50, which can have a negative impact on the bone density of hyperthyroidism patients. In this context, hyperthyroidism patients have a greater risk of osteoporosis, which may be associated with the destruction of the subchondral bone microstructure; this may be a potential mechanism by which hyperthyroidism increases the risk of OA. Chen et al.'s cross-sectional study showed that conventional indicators of thyroid function (such as TSH and TH levels) cannot be independently used as predictors of the risk of OA47, but thyroid hormone indices, such as the thyroxine resistance index (TT4RI), thyroid feedback quantitative-based index (TFQI), and free triiodothyronine/free thyroxine (FT3/FT4) ratio, should be used. The findings of this study indicated that the FT3/FT4 ratio, TT4RI, and TFQI are closely related to the development of OA47, and the authors believe that there is a correlation between the thyroid system and chondrogenic differentiation, but that study does not provide information about the underlying mechanism involved. Although our study revealed a causal relationship between hyperthyroidism and OA, the pathological mechanism underlying the relationship between hyperthyroidism and OA is still unclear. Considering that hyperthyroidism is the cause of OA, future research needs to further clarify the possible pathogenic mechanisms, which may be a breakthrough point in discovering therapeutic targets for OA.
This study has the following limitations. First, due to the lack of thyroid function measurement data for some participants in the three survey cycles, we had to exclude these participants based on the scope of this study. Due to the absence of these samples, there may be bias in the conclusions of the cross-sectional analysis. Because the target population of this study included both males and females, menopause in females was not considered a covariate, and further exploration of the effect of menopause on hyperthyroidism and OA risk in the female population is needed in future research. Second, importantly, the target population of the cross-sectional and MR analyses was individuals living in the United States and Europe, which may make the conclusions of the study unsuitable for extrapolation to populations from other countries or continents. Thus, future research needs to include correlation and genetic-level causal analysis within the same sample population, which will provide additional scientific evidence for this topic. Finally, the MR study used aggregated GWAS data and lacked individual data, which made it impossible for us to conduct hierarchical analysis and make comparisons with NHANES population data. Therefore, future MR studies should include further analysis of the risk of hyperthyroidism and OA in the population aged 60–80 years.
Conclusions
In conclusion, our cross-sectional and MR analyses showed that hyperthyroidism status increased the risk of OA, and this conclusion was reliable. The findings of this study will be beneficial for promoting the development of drug targets for OA and strengthening the monitoring of OA risk in hyperthyroidism patients. The pathological mechanism by which hyperthyroidism increases the risk of OA still needs to be clarified in future research.
Data availability
The datasets generated and analyzed in the cross-sectional study are available at NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.
Abbreviations
- OA:
-
Osteoarthritis
- NHANES:
-
National health and nutrition examination survey
- GWAS:
-
Genome wide association study
- MR:
-
Mendelian randomization
- LOO:
-
Leave-one-out sensitivity test
- IVW:
-
Inverse-variance weighting
- WME:
-
Weighted median estimator
- ASIR:
-
Age standardized incidence rate
- TSH:
-
Thyroid-stimulating hormone
- TH:
-
Thyroid hormones
- CDC:
-
Centers for disease control and prevention
- MEC:
-
Mobile examination center
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- SNPs:
-
Single nucleotide polymorphisms
- T3:
-
Triiodothyronine
- T4:
-
Thyroxine
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- CHD:
-
Coronary heart disease
- IVs:
-
Instrumental variables
References
He, M. et al. Metformin prevents or delays the development and progression of osteoarthritis: New insight and mechanism of action. Cells 11, 3012. https://doi.org/10.3390/cells11193012 (2022).
Peat, G. & Thomas, M. J. Osteoarthritis year in review 2020: epidemiology & therapy. Osteoarthr. Cartil. 29, 180–189. https://doi.org/10.1016/j.joca.2020.10.007 (2021).
Solanki, P. et al. Association between weight gain and knee osteoarthritis: a systematic review. Osteoarthr. Cartil. 31, 300–316. https://doi.org/10.1016/j.joca.2022.10.023 (2023).
Dainese, P. et al. Associations between markers of inflammation and altered pain perception mechanisms in people with knee osteoarthritis: A systematic review. RMD Open 9, e2945. https://doi.org/10.1136/rmdopen-2022-002945 (2023).
Hawker, G. A. & King, L. K. The burden of osteoarthritis in older adults. Clin. Geriatr. Med. 38, 181–192. https://doi.org/10.1016/j.cger.2021.11.005 (2022).
Jin, Z. et al. Incidence trend of five common musculoskeletal disorders from 1990 to 2017 at the global, regional and national level: Results from the global burden of disease study 2017. Ann. Rheum. Dis. 79, 1014–1022. https://doi.org/10.1136/annrheumdis-2020-217050 (2020).
Deprouw, C. et al. Pollutants: A candidate as a new risk factor for osteoarthritis—results from a systematic literature review. RMD Open 8, e1983. https://doi.org/10.1136/rmdopen-2021-001983 (2022).
Fawole, H. O. et al. Determinants of generalized fatigue in individuals with symptomatic knee osteoarthritis: The MOST study. Int. J. Rheum. Dis. 23, 559–568. https://doi.org/10.1111/1756-185X.13797 (2020).
Heijman, M. W. J. et al. Day-to-day fluctuations of fatigue severity in individuals with knee osteoarthritis: an ecological momentary assessment approach. Rheumatol. Adv. Pract. 7, rkad056. https://doi.org/10.1093/rap/rkad056 (2023).
Resende, V. A. C. et al. Higher age, female gender, osteoarthritis and blood transfusion protect against periprosthetic joint infection in total hip or knee arthroplasties: a systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 29, 8–43. https://doi.org/10.1007/s00167-018-5231-9 (2021).
Hellevik, A. I. et al. Incidence of total hip or knee replacement due to osteoarthritis in relation to thyroid function: A prospective cohort study (The Nord-Trøndelag health study). BMC Musculoskelet. Disord. 18, 201. https://doi.org/10.1186/s12891-017-1565-6 (2017).
Kim, B. Y., Kim, S., Park, H. K. & Kim, H. Assessment of the relationship between knee ultrasound and clinical symptoms in patients with thyroid dysfunction. J. Int. Med. Res. 48, 1410459914. https://doi.org/10.1177/0300060519897701 (2020).
Krieger, C. C., Neumann, S. & Gershengorn, M. C. TSH/IGF1 receptor crosstalk: Mechanism and clinical implications. Pharmacol Ther 209, 107502. https://doi.org/10.1016/j.pharmthera.2020.107502 (2020).
Chen, S., Sun, X., Zhou, G., Jin, J. & Li, Z. Association between sensitivity to thyroid hormone indices and the risk of osteoarthritis: an NHANES study. Eur J Med Res 27, 114. https://doi.org/10.1186/s40001-022-00749-1 (2022).
Xin, W. et al. Thyroid-stimulating hormone stimulation downregulates autophagy and promotes apoptosis in chondrocytes. Endocr J 64, 749–757. https://doi.org/10.1507/endocrj.EJ16-0534 (2017).
Pasqualetti, G., Pagano, G., Rengo, G., Ferrara, N. & Monzani, F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J. Clin. Endocrinol. Metabol. 100, 4240–4248. https://doi.org/10.1210/jc.2015-2046 (2015).
Wiersinga, W. M., Poppe, K. G. & Effraimidis, G. Hyperthyroidism: aetiology, pathogenesis, diagnosis, management, complications, and prognosis. Lancet Diabetes Endocrinol. 11, 282–298. https://doi.org/10.1016/S2213-8587(23)00005-0 (2023).
Cetners for disease control and prevention. about the National health and nutrition examination survey. NHANES. https://www.cdc.gov/nchs/nhanes/index.htm. Accessed 19 Jun 2023.
National Center for Health Statistics. Centers for Disease Control and Prevention NCHS research ethics review board (ERB) approval. https://www.cdc.gov/nchs/nhanes/irba98.htm. Accessed 10 Jun 2023.
Huang, J. et al. Association between lipid accumulation products and osteoarthritis among adults in the United States: A cross-sectional study, NHANES 2017–2020. Prev Med 180, 107861. https://doi.org/10.1016/j.ypmed.2024.107861 (2024).
Carlson, S. R., Imam, N., Seidenstein, A. & Klein, G. Evaluation of weight loss counsel for Osteoarthritis patients: A cross-sectional analysis of NHANES 2011–2018. Osteoarthr. Cartil. 32, 82–92. https://doi.org/10.1016/j.joca.2023.08.013 (2024).
Kakigi, C. et al. Hypothyroidism and glaucoma in the United States. Plos One 10, e133688. https://doi.org/10.1371/journal.pone.0133688 (2015).
Airaksinen, J. et al. Subclinical hypothyroidism and symptoms of depression: Evidence from the national health and nutrition examination surveys (NHANES). Compr. Psychiatry 109, 152253. https://doi.org/10.1016/j.comppsych.2021.152253 (2021).
Xing, W. et al. Sex-specific effect of urinary metabolites of polycyclic aromatic hydrocarbons on thyroid profiles: Results from NHANES 2011–2012. Environ. Sci. Pollut. Res. Int. 30, 47168–47181. https://doi.org/10.1007/s11356-023-25693-3 (2023).
University of Washington Medical Center Department of Laboratory Medicine, Immunology Division. Laboratory procedure manual: thyroid stimulating hormone. Vol 20192006. Available at: wwwn.cdc.gov/nchs/data/nhanes/2007–2008/labmethods/thyrod_e_met_thyroid_stimulating_hormone.pdf. Accessed 19 Jun 2023.
Ross, D. S. et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 26, 1343–1421. https://doi.org/10.1089/thy.2016.0229 (2016).
Thavaraputta, S., Dennis, J. A., Laoveeravat, P., Nugent, K. & Rivas, A. M. Hypothyroidism and its association with sleep apnea among adults in the United States: NHANES 2007–2008. J. Clin. Endocrinol. Metab. 104, 4990–4997. https://doi.org/10.1210/jc.2019-01132 (2019).
Rattan, P. et al. Inverse association of telomere length with liver disease and mortality in the US population. Hepatol. Commun. 6, 399–410. https://doi.org/10.1002/hep4.1803 (2022).
Grunberger, G. et al. American association of clinical endocrinology clinical practice guideline: The use of advanced technology in the management of persons with diabetes mellitus. Endocr. Pract. 27, 505–537. https://doi.org/10.1016/j.eprac.2021.04.008 (2021).
Rovin, B. H. et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 100, S1-276. https://doi.org/10.1016/j.kint.2021.05.021 (2021).
Staff, News & News, A. AAFP issues new clinical practice guideline on hypertension. Ann. Fam. Med. 21, 190–91. https://doi.org/10.1370/afm.2972 (2023).
Chen, C., Chen, K., Hsu, C., Chiu, W. & Li, Y. J. A guideline-based decision support for pharmacological treatment can improve the quality of hyperlipidemia management. Comput. Methods Progr. Biomed. 97, 280–285. https://doi.org/10.1016/j.cmpb.2009.12.004 (2010).
Gartman, E. J. et al. Summary for clinicians: Clinical practice guideline on pharmacologic management of chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 18, 11–16. https://doi.org/10.1513/AnnalsATS.202007-880CME (2021).
Kianoush, S. et al. An update on the utility of coronary artery calcium scoring for coronary heart disease and cardiovascular disease risk prediction. Curr. Atheroscler. Rep. 18, 13. https://doi.org/10.1007/s11883-016-0565-6 (2016).
Gill, D., Efstathiadou, A., Cawood, K., Tzoulaki, I. & Dehghan, A. Education protects against coronary heart disease and stroke independently of cognitive function: Evidence from Mendelian randomization. Int. J. Epidemiol. 48, 1468–1477. https://doi.org/10.1093/ije/dyz200 (2019).
NHANES Tutorials. https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx. Accessed 19 Jun 2023.
Chen, Z., Chen, Z. & Jin, X. Mendelian randomization supports causality between overweight status and accelerated aging. Aging Cell 22, e13899. https://doi.org/10.1111/acel.13899 (2023).
Chen, C. et al. Asthma and atopic dermatitis as risk factors for rheumatoid arthritis: A bidirectional mendelian randomization study. BMC Med. Genom. 16, 41. https://doi.org/10.1186/s12920-023-01461-7 (2023).
Carter, A. R. et al. Mendelian randomisation for mediation analysis: Current methods and challenges for implementation. Eur. J. Epidemiol. 36, 465–478. https://doi.org/10.1007/s10654-021-00757-1 (2021).
Drummond, S. P. et al. The recombinant Link module of human TSG-6 suppresses cartilage damage in models of osteoarthritis: A potential disease-modifying OA drug. Osteoarthr. Cartil. https://doi.org/10.1016/j.joca.2023.05.013 (2023).
Wu, J. et al. Ultra-small superparamagnetic iron oxide nanoparticles for intra-articular targeting of cartilage in early osteoarthritis. Regen. Biomater. https://doi.org/10.1093/rb/rbad052 (2023).
Rim, Y. A., Nam, Y. & Ju, J. H. The role of chondrocyte hypertrophy and senescence in osteoarthritis initiation and progression. Int. J. Mol. Sci. 21, 2358. https://doi.org/10.3390/ijms21072358 (2020).
Dequeker, J., Boonen, S., Aerssens, J. & Westhovens, R. Inverse relationship osteoarthritis-osteoporosis: what is the evidence? What are the consequences?. Br. J. Rheumatol. 35, 813–818. https://doi.org/10.1093/rheumatology/35.9.813 (1996).
Tokgöz, M. A. Is it possible that the pathogenesis of osteoarthritis could start with subchondral trabecular bone loss like osteoporosis?. Jt. Dis. Relat. Surg. 29, 152–158. https://doi.org/10.5606/ehc.2018.007 (2018).
De Berardinis, L. et al. Is primary bone marrow edema of the knee associated with thyroid disorders? A retrospective clinical study. J. Clin. Med. 11, 5973. https://doi.org/10.3390/jcm11195973 (2022).
Tsevis, K. et al. The influence of thyroid disorders on bone density and biochemical markers of bone metabolism. Horm. Mol. Biol. Clin. Investig. 35, 20180039. https://doi.org/10.1515/hmbci-2018-0039 (2018).
Chen, S. et al. Association between sensitivity to thyroid hormone indices and bone mineral density in US males. Int. J. Endocrinol. 2022, 1–10. https://doi.org/10.1155/2022/2205616 (2022).
Bernal, J. Thyroid hormone regulated genes in cerebral cortex development. J. Endocrinol. 232, R83-97. https://doi.org/10.1530/JOE-16-0424 (2017).
Nicholls, J. J., Brassill, M. J., Williams, G. R. & Bassett, J. H. D. The skeletal consequences of thyrotoxicosis. J. Endocrinol. 213, 209–221. https://doi.org/10.1530/JOE-12-0059 (2012).
Zhu, X. et al. A systematic review of the relationship between normal range of serum thyroid-stimulating hormone and bone mineral density in the postmenopausal women. BMC Women’s Health 23, 358. https://doi.org/10.1186/s12905-023-02488-9 (2023).
Acknowledgements
The authors wish to acknowledge the authors and participants of the involved GWAS for providing summary statistics data.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82305263), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515220131, No.2023A1515012626, No.2022A1515010385, No. 2022A1515011700), the National key research and development program (2021YFC1712804), Special project of State Key Laboratory of Dampness Syndrome of Chinese Medicine & the Project of Administration of Traditional Chinese Medicine of Guangdong Province (No.20225025), Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (No. YN2023MB01, YN2022GK05, No. YN2019ML08), and Health Appropriate Technology Promotion Project of Guangdong Province (No.202303211022372094).
Author information
Authors and Affiliations
Contributions
Conception and design of the work: J.Z., J.L., and L.Z. Acquisition, analysis, or interpretation of data: J.Z., H.L., M.L., G.L., L.Z. Drafting the work or substantial revision of the manuscript: J.Z., W.Y., K.H.. J.Z., H. L., and G.L. contributed equally to this work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, J., Liang, H., Liang, G. et al. Hyperthyroidism increases the risk of osteoarthritis in individuals aged 60–80 years. Sci Rep 14, 13924 (2024). https://doi.org/10.1038/s41598-024-64676-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64676-3
- Springer Nature Limited