Abstract
Fibrosis is an important complication in inflammatory bowel diseases. Previous studies suggest an important role of matrix Gla protein (MGP) and thrombospondin 2 (THBS2) in fibrosis in various organs. Our aim was to analyse their expression together with regulatory miRNAs in submucosal and subserosal fibroblasts in ulcerative colitis (UC) and Crohn’s disease (CD) using immunohistochemistry and qPCR. Digital pathology was used to compare collagen fibre characteristics of submucosal and subserosal fibrosis. Immunohistochemistry showed expression of MGP, but not THBS2 in submucosa in UC and CD. In the subserosa, there was strong staining for both proteins in CD but not in UC. qPCR showed significant upregulation of THBS2 and MGP genes in CD subserosa compared to the submucosa. Digital pathology analysis revealed higher proportion of larger and thicker fibres that were more tortuous and reticulated in subserosal fibrosis compared to submucosal fibrosis. These results suggest distinct fibroblast populations in fibrostenosing CD, and are further supported by image analysis showing significant differences in the morphology and architecture of collagen fibres in submucosal fibrosis in comparison to subserosal fibrosis. Our study is the first to describe differences in submucosal and subserosal fibroblast populations, contributing to understanding of the pathogenesis of fibrostenosis in CD.
Similar content being viewed by others
Introduction
Intestinal fibrosis is a frequent complication with significant morbidity in inflammatory bowel diseases (IBD)1. It is especially significant in Crohn`s disease (CD) where it frequently cause intestinal strictures that requires surgical intervention1,2. Although fibrosis is less prominent and with less clinical impact in ulcerative colitis (UC), colon stiffening is often seen in longstanding UC while fibrotic strictures are very rare3,4. Fibrosis as well as inflammation in CD involves the entire thickness of the bowel wall, most notably with fibrosis in the submucosa and subserosa. In UC, however, fibrosis and inflammation are generally limited to the mucosa and submucosa5.
The main effector cell of fibrosis in the bowel and other organs is the activated myofibroblast6. There are many postulated origins of activated myofibroblasts with evidence pointing to origins from residential fibroblasts, pericytes, circulating mesenchymal cells and epithelial and endothelial cells via epithelial/endothelial-mesenchymal transition7. Among organs where fibrosis has been thoroughly studied, skin is comparable to the intestinal wall as both represent a barrier separating the body from the environment and both consist of different histological layers. In the skin, there are well characterised fibroblast subpopulations with microanatomical stratification by depth8,9,10,11. They are mainly divided into papillary (more superficial) and reticular (deep) fibroblasts8,9,10,11. In recent years, some studies have identified distinct fibroblast subpopulations in the bowel wall as well; however, they focused mainly on fibroblast subpopulations in the lamina propria and submucosa12,13,14.
The gastrointestinal tract is frequently exposed to insults that can cause superficial damage such as infections, toxins and immune reactions15,16. To maintain bowel functionality, the response to such damage should be without significant scarring. However, damage that extends beyond the muscularis propria threatens the bowel wall integrity and can lead to perforation, which is a life-threatening event. In this case, fibrosis with scar formation is needed and represents a strong protective response17. Besides fibrosis, a defining characteristic of CD compared to UC is transmural inflammation, which traverses the muscularis propria into the subserosa, subsequently, providing an injury stimulus beyond the muscularis propria5. In our previous study, we identified six genes linked to fibrosis in the liver, kidney and IBD18. Two of these genes, MGP and THBS2, showed a particularly strong association with fibrostenosing CD. Matrix Gla protein (MGP) and CD36 (thrombospondin 2 (THBS2) receptor)19,20 are also markers that are differentially expressed in papillary and reticular fibroblasts of the skin21. Based on our previous evidence of MGP and THBS2 involvement in fibrosis in IBD, we decided to study them in different layers of the bowel wall to see if there is a similar stratification as observed in the skin. We hypothesized that subserosa in the bowel wall contains a fibroblastic subpopulation, which produces a strong fibrotic response to inflammatory stimuli that reach the subserosa and is distinct from the fibroblast populations of the lamina propria and submucosa. MGP and THBS2 expression is regulated by several miRNAs, among them are hsa-miR-135b22, hsa-miR-203a-3p23,24, hsa-miR-221-3p25,26,27 and hsa-miR-124628,29, which target THBS2, and hsa-miR-14330 and hsa-miR-155-5p31,32,33, which target MGP.
The aim of this study was to analyse fibroblasts in UC and CD, including immunohistochemical labelling of MGP and THBS2 protein as well as qPCR quantification of THBS2 and MGP gene expression with their regulatory miRNAs in the submucosa and subserosa. Additionally, we used a digital pathology analysis to compare the collagen fibre characteristics of subserosal and submucosal fibrosis.
Methods
Patients
This exploratory observational retrospective study was performed on formalin-fixed-paraffin-embedded tissue samples taken from surgical resection specimens of the colon and ileum of 33 patients. There were 10 patients with CD (ileal and ileocecal resections), 12 patients with UC (colon resections) and 11 patients in the control group (resected for adenocarcinoma). Samples of CD were obtained from regions with most severe stenosis, samples of UC from regions with most active inflammation and samples for the control group were obtained from morphologically normal colon. The samples, primarily used for routine histopathological examination, were collected from the archives of the Institute of Pathology, Medical Faculty, University of Ljubljana.
After surgery, the resection specimens were transported fresh to the Institute of Pathology. At the Institute of Pathology, the resection specimens were opened longitudinally, rinsed and pined on polystyrene foam boards and fixed in 10% formaldehyde for 24 h. After fixation, the specimens were sampled for routine histological analysis. The major demographic data of the patient groups included in the study are summarized in Table 1.
Ethics
This study was approved by the National Medical Ethics Committee of the Republic of Slovenia (No. 0120-139/2019/4) and performed in accordance with the guidelines of the Declaration of Helsinki.
Immunohistochemistry
For immunohistochemistry, we used antibodies against THBS2 (rabbit polyclonal, Abcam, UK, dilution 1/30), MGP (clone OTI11G6, Invitrogen, USA, dilution 1/1000), fibroblast activation protein (FAP) (clone EPR20021, Abcam, UK, dilution 1:100) and α-smooth muscle actin (α-SMA) (clone MA1-26017, Labvision, UK, dilution 1:20). Sections were treated with biotinylated secondary antibody and incubated with peroxidase conjugated streptavidin (iVIEW DAB Detection Kit, Ventana Medical System, Tucson, AZ, USA). Visualization was carried out with 3.3’ diaminobenzidine and counterstained with hematoxylin.
Immunohistochemistry quantification
THBS2 and MGP expression in submucosal and subserosal fibroblasts was quantified by adapting the method described by Qiu et al.34. Intensity and percentage of positive fibroblasts were evaluated separately in the submucosa and subserosa of each tissue section. The percentage of positive fibroblasts was determined and assigned to one of five distribution categories: 0, < 5%; 1, 5–24%; 2, 25–49%; 3, 50–74%; 4, 75–100%. The intensity of staining was scored as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). The resulting distribution category and staining score were multiplied to produce a weighted intensity distribution score (0–12). THBS2 staining intensity was scored relative to smooth muscle as the internal reference (strong staining in the smooth muscle cells). MGP staining intensity was scored relative to endothelial cells as the internal reference (strong staining in the endothelial cells).
Total RNA isolation
To compare the expression of fibrosis-related target mRNA and miRNA between submucosal and subserosal layers of the colon, the representative samples with well-characterised layers were selected. Using a 0.6 mm needle, 8–10 tissue samples of each layer were punched from tissue blocks. Total RNA was isolated using the MagMax FFPE DNA/RNA Ultra kit (A31881, Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer protocol with the following amendment: protease digestion was performed overnight at 55°C with 15s shaking at 300 rpm every 4 min. The RNA quality was assessed using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific) while the RNA concentration was determined using a Qubit Fluorimetric Quantification (Thermo Fisher Scientific) following their respective manufacturer protocols. Isolated total RNA was stored at -80°C.
Quantitation of mRNA
Reverse transcription (RT) PCR
The OneTaq RT-PCR Kit (New England Biolabs, Ipswich, MA, USA) was used to synthesize cDNA following the manufacturer protocol. Briefly, a mixture of 3 µl total RNA (60 ng) and 1 µL Random Primer Mix were incubated at 70 °C for 5 min. Then 5.0 μL of M-MuLV Reaction Mix and 1.0 μL of M-MuLV RT-ase were added. The final 10 μL RT reaction volume was then incubated at 25 °C for 5 min, at 42 °C for 1h and at 80 °C for 4 min. cDNA was immediately preamplified and then stored at − 20 °C.
cDNA preamplification
Synthesized cDNA was preamplified using the TaqMan PreAmp master mix (4391128, Applied Biosystems; Thermo Fisher Scientific) following the manufacturer protocol. Briefly, a 10 μL preamplification reaction contained 2.5 μL cDNA, 5 μL 2× TaqMan PreAmp master mix and 2.5 μL 0.2× pooled TaqMan Gene Expression Assays (4331182 and 4351372, Thermo Fisher Scientific) (Suppl. Table 1). TE buffer solution (93302-500ML, Sigma Aldrich, Burlington, MA, USA) was used to dilute the pool of TaqMan GeneExpression Assays to 0.2 × concentration. Preamplification thermocycling parameters were: 95 °C for 10 min, 10 cycles of 95 °C for 15s and 60 °C for 4 min and; finally, 99 °C for 10 min. Preamplified cDNA was stored at − 20 °C.
Quantitative Real-Time PCR (qPCR)
The expressions of IPO8, B2M, THBS2 and MGP were analysed with qPCR whereby IPO8 and B2M were used as reference genes (Suppl. Table 1). Each 10 μL qPCR reaction contained 4.5 μL of tenfold diluted preamplified cDNA, 5 μL of 2× Fast Start Essential DNA Probe Master (06924492001, Roche, Basel, Switzerland) and 0.5 μL of 20× TaqMan Gene Expression Assays (Suppl. Table 1). qPCR thermocycling parameters were: 50 °C for 2 min, 95 °C for 10 min and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Each qPCR reaction was performed in duplicates on Rotor-Gene Q System (Qiagen GmbH, Hilden, Germany).
qPCR reaction efficiency was determined using serial dilutions of pooled RNA samples for each group under the above-described conditions. Dilutions used were 5-, 10-, 25-, 125- and 625-fold. Each qPCR reaction to determine efficiency was performed in triplicates.
Quantitation of miRNA
Reverse transcription (RT) PCR
miRCURY LNA RT Kit (Qiagen GmbH) was used to synthesize cDNA. A 10 μL RT reaction contained 2 μL total RNA (10 ng), 0.5 μL UniSp6 spike-ins, 1 μL 10× miRCURY RT Enzyme Mix, 2 μL 5× miRCURY RT Reaction Buffer and 4.5 μL RNase-free water. Each RT reaction was incubated at 42 °C for 60 min and at 95 °C for 5 min. If possible, cDNA was used immediately or it was aliquoted and stored at − 20 °C.
Quantitative real-time PCR (qPCR)
The expressions of let-7e-5p, SNORD38B, miR-484, miR-221-3p, miR-1246, miR155-5p and miR-143-3p were analysed with qPCR whereby let-7e-5p, SNORD38B and miR-484 were used as reference miRNA (Suppl. Table 2). For qPCR of miRNA, miRCURY SYBR® Green PCR Kit (339347, Qiagen GmbH) was used according to manufacturer protocol. Briefly, each 10 μL qPCR reaction contained 3 μL of 60-fold diluted cDNA, 5 μL of 2× miRCURY SYBR® Green Master Mix SYBR® Green Master Mix, 1 μL 200× ROX Reference Dye and 1 μL 10× miRCURY LNA miRNA PCR Assay (339306, Qiagen GmbH) (Suppl Table 2). qPCR thermocycling parameters were: 95 °C for 2 min and 40 cycles of 95 °C for 10 s and 56 °C for 1 min. A melt curve analysis was performed in increments of 0.5 °C from 60 to 95 °C. Each qPCR reaction was performed in duplicates on QuantStudio 7 Pro (Thermo Fisher Scientific).
qPCR reaction efficiency was determined using serial dilutions of pooled RNA samples for each group under the above-described conditions. Dilutions used were 10-, 30-, 90-, 270- and 810-fold. Each qPCR reaction to determine efficiency was performed in triplicates.
Statistical analysis
All data was analysed using the IBM SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, NY). Relative gene expression was calculated using Cq averaged between duplicates of each sample. The averaged Cq values were then corrected to 100% qPCR efficiency as described by Kubista and Sindelka35. Using the method described by Latham et al.36, ΔCq was calculated by subtracting the Cq of the gene/miRNA of interest from the geometric mean Cq value of reference genes. Data distribution was tested for normality with the Shapiro–Wilk test. A paired t-test was used to determine significant differences in the expression of genes/miRNA between the submucosa and subserosa. Data homogeneity was tested using the Levene’s test. Brown-Forsythe and Welch’s ANOVA followed by Holm-Sidak or Dunnet’s T3 post-hoc analysis was used to determine significant differences between CD, UC and normal sample groups. All differences were defined as statistically significant if p < 0.05.
Digital pathology
Histological sections were stained with Masson’s trichrome with aniline blue (04-01802, Bio-Optica, Milan, Italy) for detection of fibrosis. With this stain, cytoplasm stains red, nuclei black and collagen fibres blue. Digital Pathology images are acquired at 40 × (0.23 μm/pixel) using a Hamamatsu Nanozoomer Whole Slide Imager. FibroNest™ (PharmaNest, Princeton, NJ, USA), a cloud based high-resolution single-fibre image analysis platform, was used to quantify the fibrosis phenotype for (i) collagen content and structure features (12 traits), (ii) fibre morphometry (13 traits), and (iii) fibrosis architecture (7 traits to measure the organization of the fibres)37,38. Color normalisation and standardisation39,40 was performed to calibrate the images of the study. Each trait was quantified with 7 quantitative parameters (qFTs) to account for severity, distortion and variance, resulting in a total of 315 qFTs41. Fibres were also classified into Fine (smaller and less reticulated fibres) and Assembled (complex, larger and more reticulated fibre network). The qFT dataset was automatically surveyed to identify traits (principal qFTs) that would exhibit a meaningful (> 20%) relative difference (group average) between the submucosa and subserosa groups42. Such principal qFT are normalized to the tissue area and aggregated into a normalized “Crohn-Disease” Phenotypic Fibrosis Composite Score (CD-Ph-FCS) and displayed in the form of a heat chart (Fig. 4). This automated method has superior detection thresholds to detect biologically relevant differences in the histological phenotype of fibrosis due to severity43,44,45 or etiology42.
Results
α-smooth muscle actin immunohistochemistry
In normal colon, there was staining in pericryptal myofibroblasts in the lamina propria and smooth muscle cells in muscularis mucosae, muscularis propria and vascular walls.
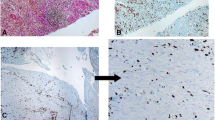
In CD, there was staining in pericryptal cells in areas without ulceration, muscularis mucosae, muscularis propria and vascular walls. Additionally, there were relatively rare positive cells in the submucosa, lamina propria cells adjacent to ulcers and erosions and many positive spindle cells in areas of fibrosis in the subserosa (Fig. 1A and E).
α-smooth muscle actin (α-SMA) and fibroblast activation protein (FAP) immunohistochemistry in Crohn’s disease and ulcerative colitis. Crohn’s diseases: α-SMA staining is present in normal structures (blood vessels and muscularis mucosae) in mucosa and submucosa (A). There is staining in muscularis propria and subserosal fibrosis (E). FAP staining is present in submucosa (B). There is staining in subserosal fibrosis but not in muscularis propria) (F). Ulcerative colitis. α-SMA staining is present in normal structures (blood vessels, muscularis mucosae, muscularis propria) (C and G). FAP staining is present in submucosa (D) but not in muscularis propria and subserosa (H).
In UC, there was staining in pericryptal cells in areas without ulceration, muscularis mucosae, muscularis propria and vascular walls. Additionally, there were relatively rare positive cells in the submucosa and lamina propria cells adjacent to ulcers and erosions. There was no staining in the subserosa outside vascular smooth muscles (Fig. 1C and G).
Fibroblast activation protein immunohistochemistry
In the normal colon, there were no FAP+ cells.
In CD, there were many FAP+ spindle shaped cells in submucosal and subserosal fibrosis and few FAP+ spindle cells in muscularis propria. In the lamina propria, there were FAP+ cells in granulation tissue beneath erosions. In the submucosa, there were many more FAP+ cells than α-SMA+ cells, while, in the subserosa, the number of FAP+ and α-SMA+ cells was comparable, and they had the same spatial distribution (Fig. 1B and F).
In UC, there were many FAP+ spindle shaped cells in submucosal fibrosis and few FAP+ spindle cells in muscularis propria. In the subserosa, there were only rare FAP+ cells in the perivascular fibrous tissue. In the lamina propria, there were FAP+ cells in granulation tissue beneath erosions. There were many more FAP+ cells in the submucosa than α-SMA+ cells (Fig. 1D and H).
Thrombospondin 2 immunohistochemistry
In the normal colon, THBS2 intensely stained the smooth muscle cells of muscularis propria and muscularis mucosae. There was also faint staining in cells corresponding to pericryptal myofibroblasts in the mucosa and rare spindle cells in the submucosa and in the outer layer of arteries.
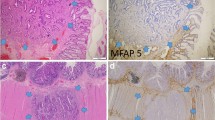
In UC, THBS2 showed a similar distribution to normal colon, with staining of smooth muscle cells and faint staining in pericryptal myofibroblasts and perivascular spindle cells. There were rare faintly stained spindle cells in areas rich with FAP+ cells in the submucosa. There were no or very few positive cells in the subserosa (Fig. 2A and E).
Thrombospondin 2 (THBS2) immunohistochemistry. No staining in submucosa in ulcerative colitis (A) and Crohn's disease (B) except in normal structures (blood vessels and muscularis mucosae). Staining of the muscularis propria and no staining in subserosa in ulcerative colitis (E). Staining of the muscularis propria and subserosal fibrosis in Crohn's disease (F). Matrix Gla protein (MGP) immunohistochemistry. Positive reaction in submucosa in ulcerative colitis (C) and Crohn's disease (D). No staining in subserosa in ulcerative colitis (G). S staining in subserosal fibrosis in Crohn's disease (H). There is staining in the blood vessel walls.
In CD, THBS2 showed a similar distribution as in UC. However, there were also numerous strongly positive cells that formed fibrosis in the subserosa, corresponding to myofibroblasts (FAP+, α-SMA+) (Fig. 2B and F).
The described patterns of staining were consistent across all studied samples (Supplementary Table 3).
Matrix Gla protein immunohistochemistry
In the normal colon, MGP stained endothelial cells, the intima of arteries, as well as scattered spindle cells in the submucosa, subserosa, and perivascular spindle cells.
In UC, MGP stained endothelial cells, the intima of arteries, as well as scattered spindle cells in the submucosa, subserosa, and in perivascular spindle cells, as in normal colon. Additionally, there was an expansion of MGP+ spindle cells in the submucosa and a focal increase of MGP+ spindle cells around blood vessels in the subserosa (Fig. 2C and G).
In CD, MGP stained endothelial cells, the intima of arteries as well as scattered spindle cells in the submucosa, subserosa, and in perivascular spindled cells. Additionally, there was an increase in MGP+ spindle cells in the submucosa as well as positive staining in the myofibroblasts forming subserosal fibrosis (Fig. 2D and H).
The described patterns of staining were consistent across all studied samples (Supplementary Table 3).
Expression of THBS2 and MGP genes as well as their corresponding miRNAs in submucosa and subserosa in Crohn’s disease, ulcerative colitis and normal colon
To determine the expression of THBS2 and MGP genes, as well as their corresponding miRNAs, we performed a qPCR analysis. Both, THBS2 and MGP, were significantly upregulated in the subserosa compared to the submucosa in CD, while only MGP was upregulated in the subserosa compared to the submucosa in UC (Fig. 3A). Interestingly, in the subserosa, only THBS2 expression was significantly higher in CD and UC compared to the normal colon (Fig. 3B). Additionally, the expression of THBS2 was significantly lower in the submucosa in CD than in the submucosa in UC (Fig. 3B).
(A) and (B) THBS2 and MGP gene expression determined by quantitative PCR. (A) THBS2 and MGP gene expression in submucosa compared to subserosa in CD (n = 10), UC (n = 12) or normal colon (n = 11). Data presented as delta delta Cq normalised to the delta Cq of respective genes in submucosa. A paired T-test was used to determine significant differences at p < 0.01 (##), p < 0.001 (###). (B) THBS2 and MGP expression profile. Data presented as the mean delta Cq ± standard deviation. One-way ANOVA with Dunnet’s T3 test were used to determine significant differences compared to the normal colon (n = 11) (*; p < 0.05) and between CD (n = 10) and UC (n = 12) (a; p < 0.05). (C) and (D) THBS2 and MGP related miRNA expression determined by quantitative PCR. (C) miR-221-3p, miR-1246, miR-155-5p, miR-143-3p expression submucosal compared to subserosa in CD (n = 10), UC (n = 12) or normal colon (n = 11). Data presented as delta delta Cq normalized to the delta Cq of respective genes in submucosa. A paired T-test was used to determine significant differences at p < 0.05 (#), p < 0.01 (##), p < 0.001 (###). (D) miR-221-3p, miR-1246, miR-155-5p, miR-143-3p expression profile. Data presented as the mean delta Cq ± standard deviation. One-way ANOVA with Holm-Sidak test were used to determine significant differences compared to the normal colon (n = 11) (*; p < 0.05, **; p < 0.01, ***; p < 0.001) and between CD (n = 10) and UC (n = 12) (a; p < 0.05, aaa; p < 0.001) or with Dunnet’s T3 test to determine significant differences compared to the normal colon (n = 11) (●; p < 0.05, ●●●; p < 0.001) and between CD (n = 10) and UC (n = 12) (b; p < 0.05). Legend CD, Crohn’s disease; UC, ulcerative colitis; N, normal colon; SM, submucosa; SS, subserosa.
Among miRNAs regulating THBS2, significant differences were observed in the expression of hsa-miR-221-3p and hsa-miR-1246 (Fig. 3C and D), while the expression hsa-miR-135b-5p and hsa-miR-203a-3p was below detectable limits. Namely, the expression of hsa-miR-221-3p was significantly lower in the subserosa compared to the submucosa in CD, while it was significantly higher in the subserosa compared to the submucosa in UC (Fig. 3C). A significantly lower expression of hsa-miR-221-3p was observed in CD compared to UC and normal colon within both the submucosa and the subserosa, while a significantly higher expression was observed only in the submucosa in UC compared to the submucosa in normal colon (Fig. 3C). On the other hand, the expression of hsa-miR-1246 was not significantly different when comparing the submucosa and the subserosa in CD, UC or normal colon (Fig. 3C). However, it was significantly lower in CD compared to UC and significantly higher in UC compared to normal colon within both the submucosa and the subserosa (Fig. 3D).
Among miRNAs regulating MGP, significant differences were observed in the expression of hsa-miR-155-5p and hsa-miR-143-3p (Figs. 3C and D). Namely, the expression of hsa-miR-155-5p was significantly lower in the subserosa compared to the submucosa in normal colon (Fig. 3C). Its expression was significantly lower in CD compared to UC and normal colon within both the submucosa and the subserosa (Fig. 3D). Additionally, the expression of hsa-miR-155-5p was significantly lower in UC compared to normal colon within both the submucosa and the subserosa (Fig. 3D). On the other hand, the expression of hsa-miR-143-3p was significantly lower in the subserosa compared to the submucosa in both CD and in normal colon (Fig. 3C). The expression of hsa-miR-143-3p was significantly higher in CD compared to UC and significantly lower in UC compared to normal colon within both the submucosa and the subserosa (Fig. 3D).
Digital pathology analysis
FibroNest Digital Pathology analysis assessed 139 principal quantitative fibrosis traits (principal qFTs) that describe the differences of the histological phenotype of fibrosis between the subserosa and the submucosa of CD fibrosis samples (Suppl Fig. 1). The related severity of each trait (green to red) is visually summarised in a phenotypic heatmap (Fig. 4A). The Phenotypic Fibrosis Composite Score formed from these principal qFTs showed statistically significant differences between the characteristics of subserosal and submucosal fibrosis (Fig. 4B). Similar composite scores can be calculated in each phenotypic layer (collagen content, fibre morphology and fibrosis architecture) (Fig. 4C–E), showing that the differences are explained by the morphometry of the fibres and their architecture, rather than the collagen content. The qFTs used to generate the composite scores describe fibre characteristics that include larger, thicker, more tortuous and reticulated fibres in the subserosa as compared to the submucosa. This novel quantitative approach is significantly superior to the conventional methods such as collagen content (Fig. 4E) that would not establish any difference.
Fibrosis phenotypic differences in fibrosis in Crohn’s disease between the submucosa and subserosa layers. (A) Phenotypic chart. Every column represents a patient, and each a principal quantitative Fibrosis Trait selected by the artificial intelligence method to identify differences. (B) Principal qFTs are combined in a Composite Phenotypic Score CD-Ph-FCS. Panels (C,D,E) represent similar composite scores for the Collagen, Fibre Morphometric and Fibrosis Architecture subphenotypes (A–E: n = 10).
Discussion
We examined the expression of THBS2 and MGP in different layers of the bowel wall in IBD on protein, mRNA and miRNA levels with the hypothesis that the fibrotic response in the bowel wall shows distinct fibroblast populations across histologic layers as seen in skin fibrosis. Activated myofibroblasts were identified immunohistochemically with antibodies against α-SMA and FAP. The former is widely used as a marker of myofibroblasts46 but it also stains smooth muscles of the muscularis mucosae and muscularis propria, while the latter stains only myofibroblasts47. On the basis of morphology and immunophenotype, we were able to distinguish between smooth muscle cells (α-SMA+, FAP−) and active myofibroblasts (α-SMA+, FAP+) in the bowel wall.
Immunohistochemical analysis of THBS2 protein expression showed no or faint staining in the submucosa in both UC and CD as well as the subserosa in UC, but intense staining in subserosal fibrosis in CD. Immunohistochemical analysis of MGP showed positive staining in submucosal fibrosis in both CD and UC as well as subserosal fibrosis of CD, but no staining in the subserosa of UC.
qPCR analysis showed a significant upregulation of THBS2 and MGP genes in the subserosa compared to the submucosa in CD. Additionally, MGP gene expression was significantly upregulated between the submucosa and the subserosa in UC, but not in the normal colon. The difference may be explained by focal areas of subserosal fibrosis in UC above the muscularis propria, which can be found in fulminant colitis48.
We also analysed the expression of regulatory miRNAs. We found downregulation of hsa-miR-221-3p in the subserosa compared to the submucosa in CD. hsa-miR-221-3p has been shown to inhibit THBS2 expression25,26,27. In UC and normal colon where there is no subserosal fibrosis, hsa-miR-221-3p downregulation in the subserosa was not observed. In fact, it was upregulated in UC. Although hsa-miR-1246 has also been shown to inhibit THBS228,29, we did not observe significant differences between the subserosa and the submucosa in any of the groups, indicating other mechanisms might have a greater role in intestinal fibrosis. We also observed downregulation of hsa-miR-143-3p, which has been shown to inhibit MGP expression30, in CD and normal subserosa compared to the submucosa. Meanwhile, the expression of hsa-miR-155-5p, another miRNA that inhibits MGP expression31,33 showed significant differences between the subserosa and the submucosa only in normal bowel, but not in CD or UC. Unexpectedly, most tested miRNAs were downregulated in UC compared to both normal colon and CD in both the subserosa and the submucosa. The only exception was hsa-miR-221-3p expression, which was not significantly different between the subserosa in normal colon and UC. In addition to THBS2 and MGP, these miRNAs have many other targets, which could potentially be of help in identifying pathways unique to UC.
Some cells in the submucosa of CD and UC were stained for α-SMA, which is a marker of smooth muscle and myofibroblasts46. However, much fewer cells were positive for α-SMA than FAP, which indicates that classical myofibroblasts represent a fraction of the expanded fibroblastic cell populations in submucosa (FAP+, α-SMA−). On the other hand, α-SMA+ cells were as abundant as FAP+ cells in the subserosa in CD, indicating that myofibroblasts represent most of the expanded fibroblastic cell populations in subserosal fibrosis (FAP+, α-SMA+).
Digital pathology analysis of the characteristics of subserosal and submucosal fibrosis in CD further confirmed that there is a significant difference in fibrosis in the two layers. The three subphenotypic scores show that the there are differences in the morphology and architecture of fibrosis in between layers, but not in collagen content. The subserosal fibrosis showed a higher proportion of more reticulated larger and thicker fibres in comparison to submucosal fibrosis, corresponding to a greater complexity of fibres and greater stiffness of the fibrosis in the subserosa45.
Myofibroblasts are widely accepted as the key cell type mediating fibrosis in various organs including the bowel wall in CD6,46; however, very little attention has been paid to the distribution of myofibroblasts in the fibrotic colon. Our study identifies activated myofibroblasts (FAP+, α-SMA+) in the subserosa indicating that injury of the subserosal compartment is necessary for the fibrostenotic reaction seen in CD.
In recent years, a microanatomically specific duality of fibroblast populations has been recognized in the skin with significant differences between papillary fibroblasts inhabiting the (superficial) papillary dermis and reticular fibroblasts inhabiting the (deeper) reticular dermis49. In skin during wound healing, reticular fibroblasts are activated first and, subsequently, mediate early extracellular matrix deposition and scar formation with loss of skin adnexa (and decreased functionality). On the other hand, skin papillary fibroblasts are activated later and mediate re-epithelization. Early activation or application of papillary fibroblasts promotes regeneration with less scar formation and regeneration of skin adnexa and functionality9,11,50,51. In wound healing and cell cultures, cells originating from reticular fibroblasts express α-SMA, while those originating from papillary fibroblasts do not8,50.
Our findings in colon show similarities to the stratification observed in the skin. We found a significant similarity in that there is a much greater expansion of fibroblasts in the subserosa than in the submucosa, with significant differences in the characteristics of the resulting fibrosis. qPCR experiments showed an upregulation of THBS2 and MGP gene expression in the subserosa of CD fibrosis, as predicted by the differences observed in the skin. Additionally, we observed differential expression of α-SMA protein between subserosal (α-SMA+) and submucosal (mainly α-SMA−) fibroblasts, again mirroring the differences observed in skin. There is no consensus on the ideal marker to differentiate papillary and reticular fibroblasts of the skin, with many other potential markers, which could show a greater difference. Based on the differences we observed between subserosal and submucosal fibroblasts, we propose that the bowel could have a similar fibroblast duality with submucosal fibroblasts activated by superficial injury mediating regeneration with little scar formation and subserosal fibroblasts activated by injury of the deeper bowel wall mediating fibrosis/scar formation. Further studies are needed to confirm this hypothesis.
Much attention has been payed to different fibroblast populations of the skin and how they could be manipulated to achieve scarless healing after injury52. As a result, the fibroblast populations of the skin are much better studied than those of the intestinal wall. If we can confirm that the intestinal wall has similar fibroblast populations, with similar behaviour, we can transfer what we have learned from the skin to the process of intestinal fibrosis, helping us understand its pathogenesis and possibly transfer therapies developed for skin fibrosis to prevent fibrostenosis in the intestine. In contrast to skin, only a few studies have focused on different fibroblast populations in the intestinal wall. Kinchen et al.13 performed single cell sequencing on endoscopic biopsy samples and identified four subpopulations of fibroblast. However, by using endoscopic biopsies they were mostly limited to the fibroblasts of the lamina propria and possibly some of the submucosa without muscularis propria or subserosa. Recently, Mukherjee et al.53 performed single cell sequencing on resection specimens. They separated the samples in mucosa/submucosa tissue and muscularis propria tissue without mention of the subserosa. Mukherjee et al. identified several distinct fibroblast subtypes in the lamina propria and muscularis propria. They observed significant differences in the lamina propria subset between stenotic and non-stenotic CD and relatively small differences in muscularis propria subsets. Shu et al.54 performed single cell sequencing on mesenteric adipose tissue and creeping fat in stenotic ileal CD. They described a novel fibroblast population, which was annotated as inflammation associated fibroblasts. There is significant anatomical overlap between creeping fat and subserosal tissue; however, it is unclear if the study included tissue involved in the stenosis or adjacent fatty tissue. Additionally, there was no comparison to the other bowel layers. They described a profibrotic and proinflammatory tendency of creeping fat fibroblasts, which could overlap with the population we describe in the subserosa. The above mentioned studies do not have enough common points to confirm if that is the case. To the best of our knowledge, our study is the first to describe a difference in fibroblast populations based on submucosal and subserosal localization.
Our study has several limitations. It is purely observational, including a limited number of patients, studying only protein and gene expression, without functional confirmation. Additionally, fibroblast markers are not very specific, except FAP, as most markers react with several other cell types. This limitation has been somewhat reduced by careful correlation with morphology and different stains. However, a study which could employ additional advanced techniques such as flow cytometry with cell sorting and single cell sequencing would produce more reliable results.
Conclusion
We observed differences in the submucosal and subserosal fibroblasts in fibrostenosing CD, suggesting distinct fibroblast populations in different layers of the bowel wall (Fig. 5). Submucosal and subserosal fibroblasts have similar markers as fibroblasts in skin in papillary and reticular dermis, respectively. Our findings provide pathogenetic explanation for the well-known fact that fibrostenosis develops in CD but not in UC: deep transmural inflammation in CD activating subserosal fibroblasts is needed to produce significant fibrosis. These results are further supported by image analysis of fibrosis in CD showing significant differences in the morphology and architecture of the collagen fibres in submucosal fibrosis in comparison to subserosal fibrosis. Our results contribute to the understanding of the pathogenesis of fibrosis in CD as well as in other diseases of the colon characterized by fibrostenosis, e.g., the diverticulitis.
Schematic representation of inflammation and fibrosis in ulcerative colitis (UC) (A) and Crohn’s disease (CD) (B). Inflammation in mucosa and submucosa in UC and CD results in mild fibrosis with fibroblasts resembling those of the papillary dermis (FAP+, αSMA − , THBS2+/ − , MGP+, while inflammation in the subserosa in CD results in extensive fibrosis with fibroblasts resembling those of the reticular dermis (FAP++, αSMA++, THBS2++, MGP++). Legend FAP, fibroblast activation protein; αSMA, α-smooth muscle actin; THBS2, thrombospondin 2; MGP, matrix Gla protein; I, mucosa; II, submucosa; III, muscularis propria; IV, subserosa.
Ethics approval statement
This study was approved by the National Medical Ethics Committee, Republic of Slovenia (No. 0120-139/2019/4) and carried out following the rules of the Declaration of Helsinki. Due to the study being done on archival tissue, the need for patient consent was waived. All authors have had access to the study data and reviewed and approved the final manuscript.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- α-SMA:
-
α-Smooth muscle actin
- CD:
-
Crohn: s disease
- FAP:
-
Fibroblast activation protein
- IBD:
-
Inflammatory bowel diseases
- MGP:
-
Matrix Gla protein
- qFT:
-
Quantitative fibrosis trait
- UC:
-
Thrombospondin 2 (THBS2), ulcerative colitis
References
Rieder, F. et al. European Crohn’s and Colitis organisation topical review on prediction, diagnosis and management of fibrostenosing Crohn’s disease. J. Crohn’s Colitis 10, 873–885 (2016).
Thia, K. T., Sandborn, W. J., Harmsen, W. S., Zinsmeister, A. R. & Loftus, E. V. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 139, 1147–1155 (2010).
Gordon, I. O., Agrawal, N., Goldblum, J. R., Fiocchi, C. & Rieder, F. Fibrosis in ulcerative colitis. Inflamm. Bowel Dis. 20, 2198–2206 (2014).
Gordon, I. O. et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment. Pharmacol. Ther. 47, 922–939 (2018).
Magro, F. et al. European consensus on the histopathology of inflammatory bowel disease. J. Crohn’s Colitis 7, 827–851 (2013).
Latella, G. & Rieder, F. Intestinal fibrosis. Curr. Opin. Gastroenterol. 33, 239–245 (2017).
Latella, G., Di Gregorio, J., Flati, V., Rieder, F. & Lawrance, I. C. Mechanisms of initiation and progression of intestinal fibrosis in IBD. Scand. J. Gastroenterol. 50, 53–65 (2014).
Janson, D. G., Saintigny, G., Van Adrichem, A., Mahé, C. & El Ghalbzouri, A. Different gene expression patterns in human papillary and reticular fibroblasts. J Invest Dermatol 132, 2565–2572 (2012).
Griffin, M. F., DesJardins-Park, H. E., Mascharak, S., Borrelli, M. R. & Longaker, M. T. Understanding the impact of fibroblast heterogeneity on skin fibrosis. Dis. Model Mech. 13 (2020).
Hu, M. S., Moore, A. L. & Longaker, M. T. A fibroblast is not a fibroblast is not a fibroblast. J Invest Dermatol 138, 729–730 (2018).
Bensa, T., Tekkela, S. & Rognoni, E. Skin fibroblast functional heterogeneity in health and disease. J. Pathol. 260, 609–620 (2023).
Powell, D. W., Pinchuk, I. V., Saada, J. I., Chen, X. & Mifflin, R. C. Mesenchymal cells of the intestinal lamina propria. Annu. Rev. Physiol. 73, 213–237 (2011).
Kinchen, J. et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 175, 372-386.e17 (2018).
Roulis, M. & Flavell, R. A. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation 92, 116–131 (2016).
Patil, D. T. & Odze, R. D. Biopsy diagnosis of colitis: an algorithmic approach. Virchows Archiv. 472, 67–80 (2018).
Jessurun, J. The differential diagnosis of acute colitis: Clues to a specific diagnosis. Surg. Pathol. Clin. 10, 863–885 (2017).
Zidar, N. Why do we have to look deep to understand diverticulitis?. Am. J. Gastroenterol. 114, 1347–1348 (2019).
Jerala, M., Hauptman, N., Kojc, N. & Zidar, N. Expression of fibrosis-related genes in liver and kidney fibrosis in comparison to inflammatory bowel diseases. Cells 11, 314 (2022).
Nan, P. et al. Tumor-stroma TGF-β1-THBS2 feedback circuit drives pancreatic ductal adenocarcinoma progression via integrin αvβ3/CD36-mediated activation of the MAPK pathway. Cancer Lett. 528, 59–75 (2022).
Simantov, R., Febbraio, M. & Silverstein, R. L. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol. 24, 27–34 (2005).
Korosec, A. et al. Lineage identity and location within the dermis determine the function of papillary and reticular fibroblasts in human skin. J. Invest. Dermatol. 139, 342–351 (2019).
Nezu, Y. et al. miR-135b, a key regulator of malignancy, is linked to poor prognosis in human myxoid liposarcoma. Oncogene 35, 6177–6188 (2016).
Qian, Z., Gong, L., Mou, Y., Han, Y. & Zheng, S. MicroRNA-203a-3p is a candidate tumor suppressor that targets thrombospondin 2 in colorectal carcinoma. Oncol. Rep. 42, 1825–1832 (2019).
Zu, F. et al. Identification of a competing endogenous RNA axis related to gastric cancer. Aging (Albany, NY). 12, 20540–20560 (2020).
Yang, Y. et al. MiR-221-3p is down-regulated in preeclampsia and affects trophoblast growth, invasion and migration partly via targeting thrombospondin 2. Biomed. Pharmacother. 109, 127–134 (2019).
Wu, X.-G. et al. Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis 22, 397–410 (2019).
Wei, W. F. et al. MicroRNA-221-3p, a TWIST2 target, promotes cervical cancer metastasis by directly targeting THBS2 article. Cell Death Disease 8, (2017).
Du, P., Lai, Y.-H., Yao, D.-S., Chen, J.-Y. & Ding, N. Downregulation of microRNA-1246 inhibits tumor growth and promotes apoptosis of cervical cancer cells by targeting thrombospondin-2. Oncol. Lett. 18, 2491–2499 (2019).
Chen, J. et al. MiR-1246 promotes SiHa cervical cancer cell proliferation, invasion, and migration through suppression of its target gene thrombospondin 2. Arch. Gynecol. Obstet. 290, 725–732 (2014).
Fiedler, J. et al. Identification of miR-143 as a major contributor for human stenotic aortic valve disease. J. Cardiovasc. Transl. Res. 12, 447–458 (2019).
Tiago, D. M., Conceição, N., Caiado, H., Laizé, V. & Cancela, M. L. Matrix Gla protein repression by MIR-155 promotes oncogenic signals in breast cancer MCF-7 cells. FEBS Lett. 590, 1234–1241 (2016).
He, X. et al. Indoxyl sulfate promotes osteogenic differentiation of vascular smooth muscle cells by miR-155-5p-dependent downregulation of matrix Gla protein via ROS/NF-κB signaling. Exp. Cell Res. 397, 112301 (2020).
Jiang, K. et al. MiR-155-5p promotes oxalate- and calcium-induced kidney oxidative stress injury by suppressing MGP expression. Oxid. Med. Cell Longev. 2020, 1 (2020).
Qiu, C.-Z. et al. Relationship between somatostatin receptor subtype expression and clinicopathology, Ki-67, Bcl-2 and p53 in colorectal cancer. World J. Gastroenterol. 12, 2011–2015 (2006).
Kubista, M. et al. The real-time polymerase chain reaction. Mol. Aspects Med. 27, 95–125 (2006).
Peltier, H. J. & Latham, G. J. Normalization of microRNA expression levels in quantitative RT-PCR assays: Identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA 14, 844–852 (2008).
Mostaço-Guidolin, L. B. et al. Collagen morphology and texture analysis: from statistics to classification. Sci. Rep. 3, 2190 (2013).
Amin, A. & Mahmoud-Ghoneim, D. Texture analysis of liver fibrosis microscopic images: A study on the effect of biomarkers. Acta Biochim. Biophys. Sin. (Shanghai) 43, 193–203 (2011).
Zheng, Y. et al. Adaptive color deconvolution for histological WSI normalization. Comput. Methods Programs Biomed. 170, 107–120 (2019).
Pérez-Bueno, F. et al. Blind color deconvolution, normalization, and classification of histological images using general super Gaussian priors and Bayesian inference. Comput. Methods Programs Biomed. 211, 106453 (2021).
Petitjean, M. M. Systems and methods for quantitative phenotyping of fibrosis. US11430112 (Patent) 2022.
Nakamura, Y. et al. Automated fibrosis phenotyping of liver tissue from non-tumor lesions of patients with and without hepatocellular carcinoma after liver transplantation for non-alcoholic fatty liver disease. Hepatol. Int. 16, 555–561 (2022).
Wang, S. et al. An autocrine signaling circuit in hepatic stellate cells underlies advanced fibrosis in nonalcoholic steatohepatitis. Sci. Transl. Med. 15, eadd3949 (2023).
Roth, J. D. et al. INT-767 improves histopathological features in a diet-induced ob/ob mouse model of biopsy-confirmed non-alcoholic steatohepatitis. World J. Gastroenterol. 24, 195–210 (2018).
Inia, J. A. et al. Semaglutide has beneficial effects on non-alcoholic steatohepatitis in Ldlr−/−. Leiden mice. Int. J. Mol. Sci. 24, 8494 (2023).
Hinz, B. et al. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am. J. Pathol. 180, 1340–1355 (2012).
Kelly, T., Huang, Y., Simms, A. E. & Mazur, A. Fibroblast activation protein-α: A key modulator of the microenvironment in multiple pathologies. Int. Rev. Cell Mol. Biol. 297, 83–116 (2012).
Odze, R. D. A contemporary and critical appraisal of ‘indeterminate colitis’. Mod. Pathol. 28, S30–S46 (2015).
Rippa, A. L., Kalabusheva, E. P. & Vorotelyak, E. A. Regeneration of dermis: Scarring and cells involved. Cells 8, 607 (2019).
Driskell, R. R. et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277–281 (2013).
Woodley, D. T. Distinct fibroblasts in the papillary and reticular dermis: Implications for wound healing. Dermatol. Clin. 35, 95–100 (2017).
Leavitt, T. et al. Scarless wound healing: Finding the right cells and signals. Cell Tissue Res. 365, 483–493 (2016).
Mukherjee, P. K. et al. Stricturing Crohn’s disease single-cell RNA sequencing reveals fibroblast heterogeneity and intercellular interactions. Gastroenterology 165, 1180–1196 (2023).
Shu, W. et al. Single-cell expression atlas reveals cell heterogeneity in the creeping fat of Crohn’s disease. Inflamm. Bowel Dis. 29, 850–865 (2023).
Acknowledgements
The authors wish to thank Eva Pavlič for Fig. 5 creation.
Funding
This work was supported by the Slovenian Research and Innovation Agency (ARIS) under research core funding number P3-0054.
Author information
Authors and Affiliations
Contributions
M.J. and N.Z. conceived the study and provided tissue samples; P.H. and N.Z. performed data collection and literature search and carried out some experiments; T.R., N.H., M.P., L.C. and M.J. carried out experiments, data analysis, and data interpretation; M.J. and N.Z. wrote the manuscript. All the authors reviewed and approved the submitted and published versions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jerala, M., Remic, T., Hauptman, N. et al. Thrombospondin 2, matrix Gla protein and digital analysis identified distinct fibroblast populations in fibrostenosing Crohn’s disease. Sci Rep 14, 13810 (2024). https://doi.org/10.1038/s41598-024-64672-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64672-7
- Springer Nature Limited