Abstract
Lignin, a heterogeneous aromatic polymer present in plant biomass, is intertwined with cellulose and hemicellulose fibrils, posing challenges to its effective utilization due to its phenolic nature and recalcitrance to degradation. In this study, three lignin utilizing bacteria, Klebsiella sp. LEA1, Pseudomonas sp. LEA2, and Burkholderia sp. LEA3, were isolated from deciduous forest soil samples in Nan province, Thailand. These isolates were capable of growing on alkali lignin and various lignin-associated monomers at 40 °C under microaerobic conditions. The presence of Cu2+ significantly enhanced guaiacol oxidation in Klebsiella sp. LEA1 and Pseudomonas sp. LEA2. Lignin-related monomers and intermediates such as 2,6-dimethoxyphenol, 4-vinyl guaiacol, 4-hydroxybenzoic acid, benzoic acid, catechol, and succinic acid were detected mostly during the late stage of incubation of Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 in lignin minimal salt media via GC–MS analysis. The intermediates identified from Klebsiella sp. LEA1 degradation suggested that conversion and utilization occurred through the β-ketoadipate (ortho-cleavage) pathway under limited oxygen conditions. The ability of these bacteria to thrive on alkaline lignin and produce various lignin-related intermediates under limited oxygen conditions suggests their potential utility in oxygen-limited processes and the production of renewable chemicals from plant biomass.
Similar content being viewed by others
Introduction
Lignocellulosic biomass is an abundant, low-cost, and renewable source for the production of biofuels and other substitutes for petroleum-derived chemicals1. The major components of lignocellulose are cellulose (40–60%), hemicellulose (20–40%), and lignin (10–25%)2. Lignin is a heterogeneous aromatic polymer, predominantly interwoven with cellulose and hemicellulose in the plant cell walls, and has been identified as a key factor impeding the effective utilization of lignocellulosic biomass. Its complex aromatic structure, along with stable carbon-to-carbon and ether linkages, confers resistance to chemical and biological degradation, thus posing challenges to decay3,4,5. Hence, the removal of lignin is crucial for improving the breakdown of plant biomass to allow access to cellulose and hemicellulose in the plant cell wall. Conventional methods for lignin removal from lignocellulose include steam explosion, organosolv treatment, and alkali or acid hydrolysis, among others6,7,8. However, these methods are associated with high costs, energy consumption, and the production of toxic phenolic compounds as undesirable byproducts5. The utilization of microorganisms in biological delignification for biomass pretreatment emerges as an ecologically friendly alternative, requiring minimal use of chemicals and energy8.
Globally, approximately 100 million tons of lignin are produced annually from lignocellulosic biomass, primarily through various pretreatment methods. The pulp and paper industry alone generates 50–70 million tons of lignin as a byproduct annually9. With the growing interest in the utilization of lignocellulosic biomass for biofuel production, there is a potential surge in annual lignin production9. Therefore, it becomes imperative to develop strategies for lignin valorization, rather than resorting to its mere combustion for fuel, as commonly practiced in pulp industries. Notably, the heterogeneous and aromatic compositions of lignin make it a potentially abundant source for various renewable or low molecular weight chemicals used in plastics production, as well as in pharmaceuticals, food, flavour, and aroma industries10,11,12. Extensive studies have demonstrated the ability of microorganisms, particularly fungi, to degrade lignin in plant structure13,14,15,16. Moreover, microbial ligninolytic enzymes have been reported to effectively decolourize aromatic organic and synthetic dyes found in wastewater effluents of textile industries17,18,19,20. Therefore, employing microorganisms to develop systems capable of efficiently breaking down lignin structures into smaller and less toxic components would be highly advantageous, not only for chemical extraction but also for remediating toxic wastes released into the environment.
Apart from fungi, lignin-degrading genes and enzymes are also present in bacteria isolated from various sources such as soil samples, rotten wood, termite guts, and coal5,12,20,21,22. Bacteria offer several advantages over fungi in the process of lignin degradation, owing to faster growth rates, broader tolerance of diverse growth conditions and habitats, facilitation of genetic manipulation, and efficient protein expression. Furthermore, large scale bacterial cultivation is potentially more efficient in terms of management17. Examples of reported bacteria and their enzymes associated with native lignin or lignin related compounds degradation include Sphingobium sp. SYK-6, Pseudomonas putida mt-2, Rhodococcus jostii RHA1, Acinetobacter sp., Streptomyces coelicolor, Microbacterium sp., Enterobacter ligninolyticus SCF1, Burkholderia sp. H1, Bacillus pumilus, and Bacillus atrophaeus6,23,24,25,26,27. Meanwhile, extensive studies on bacterial lignin degradation have been conducted under aerobic conditions, and fewer studies have explored the ability of anaerobic or facultative anaerobic bacteria to degrade lignin28,29,30. Given that limited oxygen is a common challenge in several treatment systems, bacteria capable of utilizing lignin under oxygen limitation would offer significant advantages in numerous applications.
Although different lignin-degrading bacteria have been isolated and identified, there is still a gap in the knowledge of utilization involved in lignin metabolism. The mechanisms of ligninolytic enzymes are also yet to be characterized. Application of microbial ligninolytic enzymes is still limited and not sufficient for large-scale or industries. Therefore, exploring lignin-degrading bacteria that may harbour novel or more effective enzymes for metabolizing its derivatives, particularly under oxygen limited conditions, may provide a better opportunity for improving industrial processes.
Here, the soil bacteria that display the ability to grow on alkali lignin under microaerobic conditions were isolated from tropical deciduous forest due to its richness in microbial diversity as well as the potential presence of active lignocellulolytic microorganisms31,32. Alkali lignin was used as the model to study lignin utilization in this study. Three newly identified alkali lignin utilizing bacteria were successfully isolated from soil samples collected from the deciduous forest in Nan, northern Thailand. Observations of lignin transformation in microaerobic conditions were present, and proposed pathways for the breakdown of lignin were delineated using the derived products.
Results
Bacteria isolation and identification
A total of 16 bacterial colonies with different colony sizes and morphologies were selected from the lignin enrichment process. The selected bacterial isolates were those that exhibited utilization or tolerance to the presence of phenolic compounds prevalent in lignin under limited oxygen conditions. For initial screening, colonies that grew on L-MSM (Lignin minimal salt media) with alkali lignin as the sole carbon source were selected for further screening. Afterward, the selected colonies were streaked onto carboxymethyl cellulose (CMC) agar plates to further select colonies with the ability to utilize cellulose as an energy source in addition to lignin. Nine bacterial isolates were identified by 16S rDNA sequencing to be closely related to Klebsiella sp., four were closely related to Burkholderia sp., and three to Pseudomonas sp., (Supplementary Table S1). The reported growth of the bacteria is consistent with the previous evidence in that all three genera possess lignin degrading ability33,34. Selected representative isolates, Klebsiella sp. LEA1, Pseudomonas sp. LEA2 and Burkholderia sp. LEA3 (16S rDNA sequences are available in the NCBI database with accession numbers MW186661, MW193077, and MW193078, respectively) were chosen for further characterization. The phylogenetic trees of each isolate were constructed along with the bacteria in the same genus retrieved from the NCBI database. According to the phylogenetic trees, Klebsiella sp. LEA1 is the most closely related to Klebsiella sp. FX0042 isolated from the soil in China. The bacterium is also grouped into the same clade with Klebsiella sp. P5 and K. variicola JM13 (Fig. 1A). For Pseudomonas sp. LEA2 and Burkholderia sp. LEA3, the taxonomic analysis revealed the closest relationship of the bacteria to P. aeruginosa PA0504 and B. vietnamiensis la1a4, respectively (Fig. 1B,C).
Growth on lignin monomers and effect of copper (Cu2+) on guaiacol oxidation
In preliminary experiments, Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 were able to grow on minimal salts media agar plates containing three types of model lignin monomers (guaiacol, veratryl alcohol, and 2,6-Dimethylphenol (2,6 DMP))35,36,37 as the sole carbon source, while the growth of Burkholderia sp. LEA3 was only observed on veratryl alcohol and 2,6 DMP. No growth observation was found for Burkholderia sp. LEA3 on MSM supplemented with guaiacol (Supplementary Table S2). For nutritionally rich medium, the isolates could grow on LB media supplemented with guaiacol except for Burkholderia sp. LEA3, which exhibited no growth. Copper (Cu2+) has been reported to be present at the active site of laccase enzyme38, therefore, improvement of lignin-degrading ability with the addition of Cu2+ was determined. Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 showed enhanced guaiacol oxidation with the presence of Cu2+ in the guaiacol medium (Fig. 2A). Oxidation was greatly increased when the bacteria were grown on LB based medium. Guaiacol is a major aromatic monomer found in depolymerized lignin; therefore, Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 were selected for further analyses based on the oxidation activity.
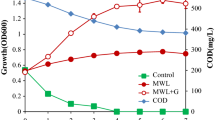
Bacterial growth and oxidation of guaiacol (A) Guaiacol oxidation with the presence of Cu2+. Effects of Cu2+ on guaiacol oxidation on minimal salt media (MSM) and LB media supplemented with 0.1% (v/v) guaiacol. Red/brown pigmentation around bacteria colonies indicates guaiacol oxidation in the media. Guaiacol oxidation experiments were carried out in triplicates (Supplementary Fig. 1). (B) Growth of Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 in L-MSM. The results of CFU/mL were calculated from triplicate samples. (C) Decrease in total phenolic content (A280) in the sample treated with Klebsiella sp. LEA1 and Pseudomonas sp. LEA2. Cultures were incubated at 40 °C for 7 days under microaerobic conditions. The absorbance of 280 nm was calculated from three independent experiments.
Growth characteristic and alkaline lignin utilization of the isolates
Growth of Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 in alkali lignin containing L-MSM was observed over a period of 7 days (Fig. 2B). Although Pseudomonas sp. LEA2 and Klebsiella sp. LEA1 were initially inoculated at an equivalent OD600 of 0.1 with slightly different inoculum (9.20 ± 0.85 × 107 and 4.52 ± 0.35 × 107 CFU/mL, respectively), Pseudomonas sp. LEA2 exhibited an accelerated growth rate in the first 3 days compared to Klebsiella sp. LEA1. Both isolates exhibited steady growth and reached their maximum cell density at day 3 of incubation, with the bacterial density of 2.29 ± 0.52 × 1011 and 4.75 ± 1.39 × 108 CFU/mL for Pseudomonas sp. LEA2 and Klebsiella sp. LEA1, respectively. The decline in growth was observed after the third day, suggesting lignin tolerance and the utilization of alkaline lignin or degraded products as a carbon source. Concurrently, the total phenolic compound was monitored through the reduction in absorbance at 280 nm (Fig. 2C). Both isolates exhibited a pronounced decrease in detected absorbance, indicating a reduction in total phenolic content. Given the potential influence of various molecules and lignin internalization by the bacteria on A280, additional experiments were subsequently conducted to conclusively support lignin-degrading ability.
The surface morphology of the lignin samples was also investigated using a scanning electron microscope (SEM) to assess the potential lignin-degrading ability of the bacteria. SEM images revealed that while control samples appeared relatively intact; samples treated with Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 exhibited rough and porous surfaces after 7 days of incubation under aerobic and oxygen limited conditions (Fig. 3), suggesting potential lignin degradation. Gel permeation chromatography (GPC) was further employed to demonstrate the potential lignin-depolymerizing ability of the bacteria. Samples of alkali lignin after a 7-day incubation with Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 in L-MSM under microaerobic conditions were extracted, and the results are summarized in Table 1. Changes in molecular weight distribution were supported by a reduction in number average molecular weight (Mn) from 791.7 ± 7.5 in the control sample to 714.0 ± 9.2 in Klebsiella sp. LEA1 and 734.3 ± 24.8 in Pseudomonas sp. LEA2. The control alkaline lignin displayed a weight average molecular weight (Mw) of 1353.7 ± 3.1 Da over a 7-day incubation period. In contrast, the samples treated with Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 exhibited a significant decrease in the Mw of alkaline lignin, reaching 1107.3 ± 20.2 Da and 1142.7 ± 44.6 Da, respectively (Fig. 4A). The shift of the peak towards the higher retention time side also demonstrated potential depolymerization of alkaline lignin by the bacterial isolates (Fig. 4B). A decrease in the polydispersity index indicates a limited molecular weight distribution of the alkaline lignin, potentially resulting from the depolymerization of large lignin molecules. Considering the observations of the reduction in Mw, Mn, along with peak shift, could suggest capacity of lignin degradation or utilization by the bacterial isolates.
SEM images of lignin samples. (A) Control sample day 0 (B) Control sample day 7 (C) Lignin sample treated with Klebsiella sp. LEA1 day 7 (microaerobic condition) (D) Lignin sample treated with Pseudomonas sp. LEA2 day 7 (microaerobic condition) (E) Lignin sample treated with Klebsiella sp. LEA1 day 7 (aerobic condition). Arrows indicate bacterial cells on lignin sample.
Analysis of alkaline lignin degradation products
GC–MS analysis was performed to identify products resulting from lignin utilization by both Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 at days 3 and 7 of incubation (Table 2, Supplementary Fig. 3). Similar intermediates and products such as 4-vinyl guaiacol, isovanillyl alcohol, and 3-vanillyl propanol were detected in both isolates. Additionally, compounds such as phenol, 3,5-dimethyl cyclopentenolone, vanilonitrile, 2,6-dimethoxyphenol, vanillyl alcohol, eicosanoic acid were exclusively detected in Pseudomonas sp. LEA2, while several unique compounds such as pentanoic acid, cinnamic acid, and 4-hydroxybenzoic acid were only observed in Klebsiella sp. LEA1. Differences in lignin-derived intermediates and products between the two isolates suggest the employment of different pathways in lignin degradation.
On day 7 of incubation under microaerobic conditions, certain organic acids such as butanoic acid, benzoic acid, and benzenepropanoic acid, which were absent on day 0 or 3 of incubation, were detected in the control samples. Although this indicates some degree of abiotic degradation in the control samples, a clear distinction was observed among the detected compounds in samples of Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 compared to the control at day 7, supporting the presence of biotic degradation in the samples (Table 2). Despite an array of intermediates and products detected in both samples on day 7, none of the isovaleric acid, butanoic acid, benzenepropanoic acid or vanillin (present in control samples) were found in the samples inoculated with either of the two bacterial isolates, suggesting the utilization of the lignin-related compounds by the bacteria. Furthermore, the presence of benzoic acid, cinnamic acid, and catechol hinted at the use of the catechol branch of the β-ketoadipate pathway (ortho-cleavage) in Klebsiella sp. LEA1 under oxygen limited conditions (Fig. 5). Different lignin intermediates were identified in samples inoculated with Pseudomonas sp. LEA2 by GC–MS analysis as mentioned earlier (Table 2). However, no obvious indicator compounds or intermediates provides guidance for the pathway likely utilized by the isolate.
Proposed lignin degradation pathways in Klebsiella sp. LEA1. The pathway with black arrows represents the proposed ortho-cleavage of catechol through the β-ketoadipate pathway in Klebsiella sp. LEA1 under microaerobic conditions. The pathway with red arrows represents the proposed degradation pathway in Klebsiella sp. LEA1 under aerobic conditions. The pathways were developed based on metabolites detected in GC–MS in this study. Lignin related compounds and metabolites detected in the GC–MS profile are marked in boxes.
The dependence of lignin-degrading enzymes on oxygen has been previously reported39. Therefore, degradation products of lignin in Klebsiella sp. LEA1 were also investigated under aerobic conditions. In contrast to the microaerobic condition, most phenolic aromatics or lignin monomers such as benzeneacetic acid, vanillin, homovanillyl alcohol, and 4-vinyl guaiacol present in the control samples were not detected in the inoculated samples by day 3 of incubation (Table S3). By day 7, guaiacol and coniferyl alcohol were absent in the sample. Interestingly, while some lignin-related compounds were no longer detected in the sample at day 7, vanillic acid was retained throughout the incubation period (Table S3). This suggests that guaiacol degradation was greatly improved under aerobic conditions.
Discussion
Lignin-degrading bacteria such as Enterobacter ligninolyticus, Burkholderia sp. H1, Bacillus pumilus, and Bacillus atrophaeus have previously been reported from soil samples6,26,27,31. Most lignin-degrading bacteria belong to three major classes: Actinomycetes, α-Proteobacteria, and γ-Proteobacteria33. Tropical forest soils have a rich microbial diversity and active lignocellulolytic microorganisms responsible for a high rate of plant litter decomposition compared to other biomes31,32,40. The genera of isolates obtained in this study (Klebsiella, Burkholderia, and Pseudomonas) showed consistency with the previous report of lignin-converting bacteria33. Alkali lignin and other model lignin compounds have generally been used to specifically isolate ligninolytic bacteria. Studies have also shown that microorganisms isolated on alkali lignin are able to degrade lignin present in plant biomass even though technical lignin varies in structure compared to native lignin5,38. The presence of these isolates in samples from different parts of Nan forest indicates their active or major involvement in lignin degradation in the environment. The ability of the isolates to grow on lignin-containing medium indicated depolymerization and their utilization of degraded compounds derived from alkaline lignin. Due to the phenolic nature of lignin, which exerts toxicity on most microorganisms by damaging cell membranes, enzymes, and nucleic acids, the ability to grow on lignin-containing media could also indicate the bacteria’s capability to tolerate lignin41. Isolates used in this study have the potential to utilize cellulose as an additional energy source based on their ability to grow on CMC plates. This is a valuable potential for lignocellulose-degrading bacteria as lignin and cellulose are major constituents of lignocellulosic biomass.
Based on the phylogenetic tree of 16S rDNA, Klebsiella sp. LEA1 shares a high sequence similarity with the soil bacterium Klebsiella sp. strain FX0042 and Klebsiella sp. strain P5 isolated from China. The isolate was also found to be closely related to the species-annotated K. variicola strain JM13 from plant rhizosphere. For Pseudomonas sp. LEA2, our bacterium shares the same node with soil isolated P. aeruginosa strain PA0504 from China. Likewise, Burkholderia sp. LEA3 is grouped with B. vietnamiensis strain la1a4 isolated from rice in Peru. The taxonomy result provides relevance to the bacteria and type of habitat, which could be further linked to the biological function.
The ability of Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 to grow on guaiacol, veratryl alcohol, and 2, 6-dimethoxyphenol (2,6-DMP) (Supplementary Table S2) suggests the presence of genes, pathways, or metabolisms that allow for oxidation of both phenolic and non-phenolic lignin monomers. The inability of Burkholderia sp. LEA3 to grow on guaiacol-supplemented media might be due to the toxicity of guaiacol42,43. Guaiacol is a predominant aromatic monomer found in depolymerized lignin44. It makes up parts of all lignin, especially softwood lignin44,45. Chow, et al.41 reported the incapability of Streptomyces sp. D7 to further degrade guaiacol after converting vanillic acid to guaiacol via decarboxylation. Enhancement of the oxidation of guaiacol by Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 was observed when copper (100 μg/mL CuSO4) was supplied in the medium (Fig. 2A).
Copper has been shown to effectively enhance the production of ligninolytic enzymes. Furthermore, numerous bacterial multi-copper oxidases or laccases have been evaluated for their ability to act on both phenolic and non-phenolic compounds38,46,47,48,49,50. Therefore, it is plausible that Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 may harbor copper-binding enzymes responsible for lignin utilization. Rezaei et al.47 reported a 13.7-fold increase in laccase production with the addition of 0.5 mM CuSO4 compared to the basal medium in Aquisalibacillus elongatus. Aside from its role in enzyme production, the supply of Cu2+ as an essential metal ion is pivotal for maintaining enzyme activity and overall lignin degradation efficiency. Copper serves as a cofactor in the active sites of laccases, facilitating the oxidation of lignin substrates51. Therefore, ensuring an adequate supply of copper ions in the growth medium is essential for maximizing the activity of ligninolytic enzymes and promoting efficient lignin degradation by bacteria.
The decline in aromatic absorbance at 280 nm indicated the reduction of the total aromatic components that occurred in the presence of Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 during the 7-day incubation period. The higher growth rate and cell density of Pseudomonas sp. LEA2 may indicate its greater tolerance to lignin or better capability of lignin depolymerization and conversion. The decline in cell growth observed after day 3 of incubation is possibly due to the accumulation of phenolic compounds and other products that can produce inhibitory effects on cell growth52.
SEM images revealed rough surfaces and pores on the surface of lignin particles treated with isolates further indicating the potential lignin degrading ability of the isolates. Similar results have been reported in other studies that treated alkali lignin with lignin degrading bacteria6,53. During the process of the SEM sample preparation, the supernatant from control samples appeared colourless while the supernatant from bacteria treated samples was brownish. This phenomenon likely arises from the erosion of the lignin surface, leading to the detachment of smaller particles that are subsequently absorbed or extracted into ethanol. A similar observation was also noticed in the ethyl acetate extracts of control and bacteria treated samples for GC–MS analysis. Extracts of uninoculated samples were colourless while those of samples treated with bacteria were yellowish in colour (Supplementary Fig. 4). GPC analysis suggests that Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 possess the capability to depolymerize lignin, resulting in the production of smaller lignin molecules. This is supported by the observed shift in the chromatogram peak, signifying a decrease in the Mw, along with the accumulation of small lignin-derived molecules. It should be noted that the alkaline lignin used in this study underwent sterilization through autoclaving, a process wherein moist heat and pressure could potentially impact the structure of the alkaline lignin54,55. Therefore, we cannot rule out the possibility that the two isolates may also utilize the molecules degraded through abiotic degradation. Future studies aimed at assessing the utilization of small phenolic compounds, such as HPLC, could enhance precision in evaluating the degradation of lignin.
Many lignin-derived intermediates and products were detected by GC–MS analysis by day 7. It appears that most degradation products were formed or accumulated at the latter stage of incubation. Orellana, et al.56 reported that most up-regulation of ligninolytic enzymes such as laccase, peroxidases, and aryl-alcohol dehydrogenases occurred during the mid-exponential and early stationary growth phase in Enterobacter lignolyticus SCF1. Weinstein, et al.57 also reported the degradation of β-guaiacyl ether-linked lignin dimeric compounds during the stationary growth phase of Phanerochaete chrysosporium. Lignin-related compounds detected by GC–MS throughout 7 days of incubation under microaerobic conditions suggest that Klebsiella sp. LEA1 possibly used the catechol branch of the β-ketoadipate pathway for the metabolism of aromatic compounds obtained from alkali lignin (Fig. 5). Several lignin-related monomers such as cinnamic acid, catechol, benzoic acid and 4-hydroxybenzoic acid, which can be further degraded through the gentisate or β-ketoadipate pathways were detected after 7 days of incubation58,59,60. The presence of succinic acid (butanedioic acid) in the degrading samples suggests mineralization by ortho-cleavage of catechol through the β-ketoadipate pathway before finally entering the tricarboxylic acid (TCA) cycle for an energy source in Klebsiella sp. LEA161,62. Our proposed mechanism of Klebsiella sp. LEA1 was in accordance with the genomic analysis of Klebsiella pneumoniae AWD5 which harbors an ortho-cleavage pathway for polyaromatic hydrocarbon degradation63. Although the metabolic pathway employed by Pseudomonas LEA2 cannot be established in this study, it is evident that the isolate is capable of utilizing lignin-based compounds, as inferred from the GC–MS and GPC analysis. Previous studies have demonstrated the coexistence of both protocatechuate and catechol branches within the β-ketoadipate pathway in Pseudomonas aeruginosa which are selectively used depending on the compounds or intermediates the bacterium is exposed to64,65.
Under aerobic incubation, most lignin-related compounds present in the samples were completely degraded by day 7 suggesting that oxygen could accelerate the degradation process in Klebsiella sp. LEA1. A previous study on Phanerochaete chrysosporium revealed enhancement of lignin dimer metabolism at increased oxygen concentrations suggesting the oxidative nature of steps involved in lignin degradation25. Vanillic acid has been identified as one of the major aromatic metabolites obtained from different intermediates and enzymatic reactions involved in lignin degradation. Vanillic acid can also be further degraded or mineralized to simpler compounds depending on the pathway employed by the bacteria12,13,66,67. As seen in Table 2 and Table S3, vanillic acid was present in samples throughout the incubation period. Although the degradation steps after the accumulation of vanillic acid are unclear based on the GC–MS result, it is possible that ligninolytic bacteria funnel vanillic acid into protocatechuic acid (Fig. 5). Protocatechuic acid is further dearomatized through the β-ketoadipate pathway by protocatechuate cleavage before finally entering the TCA cycle12,60,67.
Previous studies in Klebsiella and Pseudomonas have reported the presence of a complete β-ketoadipate pathway through the protocatechuate and catechol routes as well as genes and enzymes involved in lignin degradation such as peroxidases, monooxygenases, and laccase63,68,69,70,71. Discrepancies observed between alkali lignin degradation patterns under aerobic and limited oxygen conditions indicate the effect of environmental factors, particularly oxygen, on lignin metabolism. As seen in the results presented here, diverse degradation products were detected under microaerobic conditions compared to aerobic conditions, while vanillic acid and vanilonitrile were the only products detected under aerobic conditions. Many of the compounds discovered under oxygen limited conditions are valuable in the food, flavour and aroma industries as well as in both pharmaceutical and cosmetic sectors12. Thus, microaerobic conditions might be recommended to obtain a wider profile of compounds from bacterial lignin degradation. Although some studies have reported the ability of bacteria to degrade lignin, most of these studies mainly focus on lignin degradation under aerobic conditions59,60. Hence, bacteria that can utilize lignin under limited oxygen conditions and elevated temperature (40 °C) would be advantageous in anoxic conditions, which are more applicable in industrial settings. Further characterization of the physiological and biochemical features of these isolates, along with an understanding of related regulatory systems, may facilitate the optimization of lignin valorization for the production of various value-added compounds, particularly under oxygen-limited conditions.
Materials and methods
Soil sampling and bacterial isolation
Soil samples were collected at 20–30 cm depth from a deciduous forest in Nan province, Thailand32. Sample collecting sites in this study located in the boundary area of the national park, no restrictions regarding permission are required. Samples were immediately kept in microaerobic conditions (CO2:5–6%; O2:6–10%) using AnaeroPack™ (Mitsubishi Gas Chemical, Japan) and stored at 4 °C for further analysis within two days. To isolate lignin degrading bacteria, the soil samples were enriched in autoclaved minimal salt media (MSM) containing 1 g/L alkali (Sigma-Aldrich) lignin (L-MSM) as the sole carbon source at 40 °C for 7 days under microaerobic conditions. The L-MSM contained (g/L) Na2HPO4 (2.4), K2HPO4 (2.0), NH4NO3 (0.1), MgSO4.7H2O (0.5), CaCl2 (0.01), MnSO4.H2O (0.02), FeSO4.7H2O (0.01), alkali lignin (1.0), pH 7.2. The L-MSM was sterilized by autoclaving before use. After the enrichment period, 1 mL of culture was serially diluted and spread on Berg’s mineral salts agar supplemented with 1% carboxymethyl cellulose (CMC) (g/L) (NaNO3 (2), K2HPO4 (0.05), MgSO4.7H2O (0.5), MnSO4.H2O (0.02), FeSO4.7H2O (0.01), CaCl2.2H2O (0.02). Isolates that appeared on CMC plates were restreaked four times to get axenic cultures and finally transferred to plating on L-MSM agar.
16S rDNA identification of isolates
Bacterial isolates that grew on L-MSM were cultured overnight in LB medium at 40 °C, with periodic shaking at 200 rpm. Genomic DNA was extracted from overnight cultures using the phenol–chloroform extraction method. The full length of 16S rDNA was amplified using universal primers 27F and 1492R (27F 5’-AGAGTTTGATCCTGGCTCAG-3’; 1492R 5’-GGTTACCTTGTTACGACTT-3’) followed by sequencing analyses. For bacterial identification, sequence homology was compared using the Basic Local Alignment Search Tool (BLAST) against the National Center for Biological Information (NCBI) database. A phylogenetic tree of 16S rDNA was constructed by the neighbor joining method, 1000 bootstrap replications using MEGA11 software.
Growth on different lignin monomers
The ligninolytic ability of selected isolates was tested using model lignin monomers (guaiacol, veratryl alcohol, and 2, 6-dimethoxyphenol (2,6-DMP)) (Sigma-Aldrich). Guaiacol and 2, 6-DMP represented phenolic substrates, while veratryl alcohol represented a non-phenolic substrate61. Preculture of bacterial isolate in LB with the OD600 of 1.0 was collected after centrifuging at 5000 × g for 5 min and washed with normal saline (0.9% NaCl) three times. The bacterial suspension was then adjusted to OD600 of 0.1 and 5 µl of the suspension was gently dropped onto MSM plates supplemented with 0.1% (v/v) guaiacol, veratryl alcohol, and 0.1% (w/v) 2, 6-DMP. The MSM plates were incubated for 7 days at 40 °C under microaerobic conditions.
Effect of copper (Cu) on lignin degradation
0.1 g/L CuSO4 was incorporated into LB agar and MSM agar supplemented with 0.1% (v/v) guaiacol to investigate the influence of copper on guaiacol oxidation. The sample inoculated with Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 were kept at 40 °C in microaerobic conditions for 7 days. Agar plates were examined for the oxidation of guaiacol indicated by the presence of pink or brown pigments around bacterial colonies.
Growth characteristic of the isolates and total phenolic content analysis
Based on the effect of copper on guaiacol oxidation, L-MSM was modified to contain 0.1 g/L CuSO4. Klebsiella sp. LEA1 Pseudomonas sp. LEA2 and were inoculated into 50 mL L-MSM at an equivalent OD600 of 0.1. Growth of the bacterial isolates in the media over time was followed at 40 °C for 7 days under microaerobic conditions. The culture suspension was sampled on days 1, 2, 3, 5, and 7 of the incubation to determine colony forming units (CFU), as the colour of alkali lignin interfered with the turbidity of the solution.
To further test for the reduction of total phenolic content, the culture supernatant was withdrawn and acidified with concentrated hydrochloric acid (HCl) to pH 2. The sample was centrifuged at 12,000 × g for 10 min and the supernatant was collected. Total phenolic content was monitored by measuring the remaining phenolic compounds at the absorbance of 280 nm using a V530 UV/Vis spectrophotometer (Jasco, Japan).
Field emission scanning electron microscope (FE-SEM)
Visual assessment of the surface morphology and structure of lignin samples was done using a field emission scanning electron microscope (FE-SEM). Two milliliters of control (no bacterium) and inoculated samples were withdrawn after 7 days and centrifuged at 12,000 × g for 10 min. The precipitates were collected and fixed in 2.5% glutaraldehyde. Precipitates were washed twice in phosphate buffer saline (PBS) pH 7 followed by sterile distilled water. The samples were dehydrated in a gradient of 25–95% ethanol and coated with a layer of Pt/Pd alloy via critical point drying and ion sputtering. Afterward, samples were imaged with FE-SEM (Hitachi SU-8010).
Gel permeation chromatography (GPC)
The molecular weight distribution of alkaline lignin was investigated using the GPC-Tetrahydrofuran (THF) system (Waters e2695, Netherlands). A total of 0.1% of alkaline lignin was incubated in a minimal salt medium with or without bacteria at 40 °C for 7 days. Alkaline lignin in the media was collected by adding 6 M HCl, centrifuged at 12,000 × g for 10 min, and dried at 60 °C. Dried lignin was acetylated using 2 mL of equal volume of acetic anhydride and pyridine. The reaction mixture was stirred for 24 h before adding 0.1 M HCl, centrifuged at 12,000 × g for 10 min, and dried at 60 °C. Dried acetylated samples were dissolved in THF and filtered through 0.2-μm nylon membrane syringe filters. Size exclusion was performed at room temperature with THF as the mobile phase (flow rate of 1 mL min–1) with RI, light scattering and viscometry detectors. The calibration was performed by using polystyrene standards with the Mw range between 1250 and 920,000 g mol−1. For GPC analysis, three independent experiments were conducted.
Gas chromatography mass spectrometry (GC–MS) analysis of lignin degradation products
Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 isolates were grown in L-MSM under microaerobic conditions for 7 days in triplicates, while the investigation under aerobic conditions was additionally done in Klebsiella sp. LEA1. Samples were collected on days 3 and 7 of incubation. Fifty millilitres of samples were centrifuged (5000 × g, 30 min) and the supernatants were acidified to pH 1–2 using 6 M HCl. After acidification, samples were centrifuged and the supernatants were harvested. Supernatants were thoroughly extracted three times with ethyl acetate and the organic layer was collected followed by dewatering over anhydrous Na2SO4 in filter paper to remove moisture. The organic layer of triplicates of each treatment was pooled together, and samples were placed in a rotary evaporator at 37 °C for further moisture removal. The residues were then resuspended in 10 mL of ethyl acetate. Two milliliters of ethyl acetate suspension were derivatized after evaporation of the solvent under nitrogen gas using 70 µL N-Methyl-N-(trimethylsilyl) trifluoroacetamide with 1% trimethylchlorosilane (MSTFA) and 40 µL Methoxamine (MOX) reagent. The mixture was heated at 40 °C for 1 h with periodic shaking.
One microliter of the sample was subjected to the GC–MS (Agilent Technologies USA 5977B MSD; 7890 BGC) for separation by HP-5MS UI capillary column (29.90 m × 0.25 mm × 0.25 µm). Helium was used as a carrier gas with a flow rate of 1 mL/min. The column was held at 50 °C for 5 min and increased to 300 °C (10 °C per min, hold time: 5 min). The transfer line and ion source temperatures were maintained at 200 and 250 °C. A solvent delay of 3.5 min was selected. Electron ionization mass spectra in the range of 30–550 (m/z) were recorded at an electron energy of 70 eV. The compounds were identified by comparing the retention times of standards and data in the National Institute of Standards and Technology (NIST) library.
Conclusion
In this study, bacteria capable of converting and utilizing alkaline lignin were isolated from soil samples collected from the forest in northern Thailand. Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 were able to grow on alkali lignin and lignin monomers under microaerobic conditions and elevated temperature (40 °C). SEM images revealed the erosion of the lignin surface by the isolates, consistent with the depolymerization ability suggested by GPC analysis. The profile of compounds detected using GC–MS indicated the mineralization of lignin monomers and the possible presence of lignin-degrading enzymes, genes, and pathways such as the gentisate pathway and the β-ketoadipate pathway in Klebsiella sp. LEA1. Our results also indicate increased alkaline lignin conversion during the late stage of the incubation period of bacterial isolates. Klebsiella sp. LEA1 and Pseudomonas sp. LEA2 possess the ability to utilize alkaline lignin under the limited oxygen conditions commonly encountered in fermentation systems. Thus, the use of these lignin-utilizing bacterium can be explored further in studies related to lignin valorization and degradation under oxygen limited conditions.
Data availability
Raw data generated and analyzed in this study are available in the depository links: Bacterial growth: https://doi.org/10.6084/m9.figshare.21814164 GC–MS: https://doi.org/10.6084/m9.figshare.21814152 SEM images: https://doi.org/10.6084/m9.figshare.21958379 GPC: https://doi.org/10.6084/m9.figshare.25901380.v1 Requests for further information or materials should be addressed to P.H.
References
Saini, J. K., Saini, R. & Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 5, 337–353. https://doi.org/10.1007/s13205-014-0246-5 (2015).
Kang, Q., Appels, L., Tan, T. & Dewil, R. Bioethanol from lignocellulosic biomass: Current findings determine research priorities. ScientificWorldJournal 2014, 298153. https://doi.org/10.1155/2014/298153 (2014).
Raj, A., Chandra, R., Reddy, M. M. K., Purohit, H. J. & Kapley, A. Biodegradation of kraft lignin by a newly isolated bacterial strain, Aneurinibacillus aneurinilyticus from the sludge of a pulp paper mill. World J. Microbiol. Biotechnol. 23, 793–799. https://doi.org/10.1007/s11274-006-9299-x (2007).
Casciello, C. et al. A valuable peroxidase activity from the novel species Nonomuraea gerenzanensis growing on alkali lignin. Biotechnol. Rep. (Amst.) 13, 49–57. https://doi.org/10.1016/j.btre.2016.12.005 (2017).
Wang, W., Yang, S., Hunsinger, G. B., Pienkos, P. T. & Johnson, D. K. Connecting lignin-degradation pathway with pre-treatment inhibitor sensitivity of Cupriavidus necator. Front. Microbiol. 5, 247. https://doi.org/10.3389/fmicb.2014.00247 (2014).
Yang, C., Wang, T., Gao, L. N., Yin, H. J. & Lü, X. Isolation, identification and characterization of lignin-degrading bacteria from Qinling, China. J. Appl. Microbiol. https://doi.org/10.1111/jam.13562 (2017).
Yang, S., Yuan, T.-Q., Li, M.-F. & Sun, R.-C. Hydrothermal degradation of lignin: Products analysis for phenol formaldehyde adhesive synthesis. Int. J. Biol. Macromol. 72, 54–62. https://doi.org/10.1016/j.ijbiomac.2014.07.048 (2015).
Kumar, P., Barrett, D. M., Delwiche, M. J. & Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 48, 3713–3729. https://doi.org/10.1021/ie801542g (2009).
Haq, I., Mazumder, P. & Kalamdhad, A. S. Recent advances in removal of lignin from paper industry wastewater and its industrial applications—A review. Bioresour. Technol. 312, 123636. https://doi.org/10.1016/j.biortech.2020.123636 (2020).
Bugg, T. D. H. & Rahmanpour, R. Enzymatic conversion of lignin into renewable chemicals. Curr. Opin. Chem. Biol. 29, 10–17. https://doi.org/10.1016/j.cbpa.2015.06.009 (2015).
Varanasi, P. et al. Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnol. Biofuels 6, 14. https://doi.org/10.1186/1754-6834-6-14 (2013).
Xu, Z., Lei, P., Zhai, R., Wen, Z. & Jin, M. Recent advances in lignin valorization with bacterial cultures: Microorganisms, metabolic pathways, and bio-products. Biotechnol. Biofuels 12, 32. https://doi.org/10.1186/s13068-019-1376-0 (2019).
Zhu, D. et al. Biodegradation of alkaline lignin by Bacillus ligniniphilus L1. Biotechnol. Biofuels 10, 44. https://doi.org/10.1186/s13068-017-0735-y (2017).
Su, Y. et al. Biodegradation of lignin and nicotine with white rot fungi for the delignification and detoxification of tobacco stalk. BMC Biotechnol. 16, 81. https://doi.org/10.1186/s12896-016-0311-8 (2016).
Zhang, S., Jiang, M., Zhou, Z., Zhao, M. & Li, Y. Selective removal of lignin in steam-exploded rice straw by Phanerochaete chrysosporium. Int. Biodeteriorat. Biodegrad. 75, 89–95. https://doi.org/10.1016/j.ibiod.2012.09.003 (2012).
Ulmer, D., Leisola, M., Schmidt, B. & Fiechter, A. Rapid degradation of isolated lignins by Phanerochaete chrysosporium. Appl. Environ. Microbiol. 45, 1795–1801. https://doi.org/10.1128/AEM.45.6.1795-1801.1983 (1983).
Verma, P. & Madamwar, D. Decolorization of synthetic textile dyes by lignin peroxidase ofPhanerochaete chrysosporium. Folia Microbiol. 47, 283–286. https://doi.org/10.1007/BF02817653 (2002).
Kunjadia, P. D., Sanghvi, G. V., Kunjadia, A. P., Mukhopadhyay, P. N. & Dave, G. S. Role of ligninolytic enzymes of white rot fungi (Pleurotus spp.) grown with azo dyes. SpringerPlus 5, 1487. https://doi.org/10.1186/s40064-016-3156-7 (2016).
López, M. J., Guisado, G., Vargas-García, M. C., Suárez-Estrella, F. & Moreno, J. Decolorization of industrial dyes by ligninolytic microorganisms isolated from composting environment. Enzyme Microb. Technol. 40, 42–45. https://doi.org/10.1016/j.enzmictec.2005.10.035 (2006).
Dineshkumar, B. et al. Bacterial lignin peroxidase: A tool for biobleaching and biodegradation of industrial effluents. Univ. J. Environ. Res. Technol. 2, 58–64 (2012).
Taylor, C. R. et al. Isolation of bacterial strains able to metabolize lignin from screening of environmental samples. J. Appl. Microbiol. 113, 521–530. https://doi.org/10.1111/j.1365-2672.2012.05352.x (2012).
Wang, L. et al. Diverse bacteria with lignin degrading potentials isolated from two ranks of coal. Front. Microbiol. 7, 1428. https://doi.org/10.3389/fmicb.2016.01428 (2016).
Wenzel, M., Schönig, I., Berchtold, M., Kämpfer, P. & König, H. Aerobic and facultatively anaerobic cellulolytic bacteria from the gut of the termite Zootermopsis angusticollis. J. Appl. Microbiol. 92, 32–40. https://doi.org/10.1046/j.1365-2672.2002.01502.x (2002).
Ahmad, M. et al. Development of novel assays for lignin degradation: Comparative analysis of bacterial and fungal lignin degraders. Mol. bioSyst. 6, 815–821. https://doi.org/10.1039/b908966g (2010).
Masai, E., Katayama, Y. & Fukuda, M. Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci. Biotechnol. Biochem. 71, 1–15. https://doi.org/10.1271/bbb.60437 (2007).
Huang, X.-F. et al. Isolation and characterization of lignin-degrading bacteria from rainforest soils. Biotechnol. Bioeng. https://doi.org/10.1002/bit.24833 (2013).
Deangelis, K. M. et al. Evidence supporting dissimilatory and assimilatory lignin degradation in Enterobacter lignolyticus SCF1. Front. Microbiol. 4, 280. https://doi.org/10.3389/fmicb.2013.00280 (2013).
Nurika, I. et al. Application of ligninolytic bacteria to the enhancement of lignocellulose breakdown and methane production from oil palm empty fruit bunches (OPEFB). Bioresour. Technol. Rep. 17, 100951. https://doi.org/10.1016/j.biteb.2022.100951 (2022).
Jing, D., Jidong, L., Yiping, W. & Wenjing, D. Kraft lignin biodegradation by Dysgonomonas sp. WJDL-Y1, a new anaerobic bacterial strain isolated from sludge of a pulp and paper mill. J. Microbiol. Biotechnol. 26, 1765–1773. https://doi.org/10.4014/jmb.1602.02014 (2016).
Duan, J. et al. Biodegradation of kraft lignin by a newly isolated anaerobic bacterial strain, Acetoanaerobium sp. WJDL-Y2. Lett. Appl. Microbiol. 62, 55–62. https://doi.org/10.1111/lam.12508 (2016).
Woo, H. L., Hazen, T. C., Simmons, B. A. & DeAngelis, K. M. Enzyme activities of aerobic lignocellulolytic bacteria isolated from wet tropical forest soils. Syst. Appl. Microbiol. 37, 60–67. https://doi.org/10.1016/j.syapm.2013.10.001 (2014).
Harnvoravongchai, P. et al. Isolation and characterization of thermophilic cellulose and hemicellulose degrading bacterium, Thermoanaerobacterium sp. R63 from tropical dry deciduous forest soil. PLOS ONE 15, e0236518. https://doi.org/10.1371/journal.pone.0236518 (2020).
Bugg, T. D., Ahmad, M., Hardiman, E. M. & Singh, R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr. Opin. Biotechnol. 22, 394–400. https://doi.org/10.1016/j.copbio.2010.10.009 (2011).
Gaur, N., Narasimhulu, K. & Pydi Setty, Y. Extraction of ligninolytic enzymes from novel Klebsiella pneumoniae strains and its application in wastewater treatment. Appl. Water Sci. 8, 111. https://doi.org/10.1007/s13201-018-0758-y (2018).
Kumar, R. et al. Effective bioremediation of pulp and paper mill wastewater using Bacillus cereus as a possible Kraft lignin-degrading bacterium. Bioresour. Technol. 352, 127076. https://doi.org/10.1016/j.biortech.2022.127076 (2022).
Pan, Y. et al. Selective conversion of lignin model veratryl alcohol by photosynthetic pigment via photo-generated reactive oxygen species. Chem. Eng. J. 393, 124772. https://doi.org/10.1016/j.cej.2020.124772 (2020).
Kang, J., Irmak, S. & Wilkins, M. Conversion of lignin into renewable carboxylic acid compounds by advanced oxidation processes. Renew. Energy 135, 951–962. https://doi.org/10.1016/j.renene.2018.12.076 (2019).
Worrall, J. & Vijgenboom, E. Copper mining in Streptomyces: Enzymes, natural products and development. Nat. Prod. Rep. 27, 742–756. https://doi.org/10.1039/b804465c (2010).
Wang, X., Yao, B. & Su, X. Linking enzymatic oxidative degradation of lignin to organics detoxification. Int. J. Mol. Sci. 19, 3373 (2018).
Parton, W. et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315, 361–364. https://doi.org/10.1126/science.1134853 (2007).
Chow, K. T., Pope, M. K. & Davies, J. Characterization of a vanillic acid non-oxidative decarboxylation gene cluster from Streptomyces sp. D7. Microbiology (Reading) 145(Pt 9), 2393–2403. https://doi.org/10.1099/00221287-145-9-2393 (1999).
García-Hidalgo, J., Ravi, K., Kuré, L.-L., Lidén, G. & Gorwa-Grauslund, M. Identification of the two-component guaiacol demethylase system from Rhodococcus rhodochrous and expression in Pseudomonas putida EM42 for guaiacol assimilation. AMB Express 9, 34–34. https://doi.org/10.1186/s13568-019-0759-8 (2019).
Yahaya, A., Ursel, H. & Idris, M. B. in Biorefinery Concepts, Energy and Products (ed Beschkov Venko) Ch. 5 (IntechOpen, 2020).
Barton, N. et al. Enabling the valorization of guaiacol-based lignin: Integrated chemical and biochemical production of cis, cis-muconic acid using metabolically engineered Amycolatopsis sp. ATCC 39116. Metab. Eng. 45, 200–210. https://doi.org/10.1016/j.ymben.2017.12.001 (2018).
Ravi, K. et al. Bacterial conversion of depolymerized Kraft lignin. Biotechnol. Biofuels 12, 56. https://doi.org/10.1186/s13068-019-1397-8 (2019).
Santo, M., Weitsman, R. & Sivan, A. The role of the copper-binding enzyme—Laccase—In the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int. Biodeteriorat. Biodegrad. 84, 204–210. https://doi.org/10.1016/j.ibiod.2012.03.001 (2013).
Ur Rahman, M. et al. Harnessing the power of bacterial laccases for xenobiotic degradation in water: A 10-year overview. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2024.170498 (2024).
Zheng, X., Ng, I. S., Ye, C., Chen, B. Y. & Lu, Y. Copper ion-stimulated McoA-laccase production and enzyme characterization in Proteus hauseri ZMd44. J. Biosci. Bioeng. 115, 388–393. https://doi.org/10.1016/j.jbiosc.2012.10.012 (2013).
Rezaei, S., Shahverdi, A. R. & Faramarzi, M. A. Isolation, one-step affinity purification, and characterization of a polyextremotolerant laccase from the halophilic bacterium Aquisalibacillus elongatus and its application in the delignification of sugar beet pulp. Bioresour. Technol. 230, 67–75. https://doi.org/10.1016/j.biortech.2017.01.036 (2017).
Khan, S. I. et al. Production and characterization of novel thermostable CotA-laccase from Bacillus altitudinis SL7 and its application for lignin degradation. Enzyme Microbial Technol. 172, 110329. https://doi.org/10.1016/j.enzmictec.2023.110329 (2024).
Granja-Travez, R. S. et al. Structural and functional characterisation of multi-copper oxidase CueO from lignin-degrading bacterium Ochrobactrum sp. reveal its activity towards lignin model compounds and lignosulfonate. FEBS J. 285, 1684–1700. https://doi.org/10.1111/febs.14437 (2018).
Li, X., Li, M., Pu, Y., Ragauskas, A. J. & Zheng, Y. Simultaneous depolymerization and fermentation of lignin into value-added products by the marine protist, Thraustochytrium striatum. Algal Res. 46, 101773. https://doi.org/10.1016/j.algal.2019.101773 (2020).
Xu, Z., Qin, L., Cai, M., Hua, W. & Jin, M. Biodegradation of kraft lignin by newly isolated Klebsiella pneumoniae, Pseudomonas putida, and Ochrobactrum tritici strains. Environ. Sci. Pollut. Res. 25, 14171–14181. https://doi.org/10.1007/s11356-018-1633-y (2018).
Gabhane, J., William, S. P. M. P., Vaidya, A. N., Mahapatra, K. & Chakrabarti, T. Influence of heating source on the efficacy of lignocellulosic pretreatment—A cellulosic ethanol perspective. Biomass Bioenergy 35, 96–102. https://doi.org/10.1016/j.biombioe.2010.08.026 (2021).
Huang, W. et al. Effect of physicochemical pretreatments and enzymatic hydrolysis on corn straw degradation and reducing sugar yield. BioResources 12, 7002–7015. https://doi.org/10.15376/biores.12.4.7002-7015 (2017).
Orellana, R. et al. Multi-time series RNA-seq analysis of Enterobacter lignolyticus SCF1 during growth in lignin-amended medium. PLOS ONE 12, e0186440. https://doi.org/10.1371/journal.pone.0186440 (2017).
Weinstein, D. A., Krisnangkura, K., Mayfield, M. B. & Gold, M. H. Metabolism of radiolabeled β-guaiacyl ether-linked lignin dimeric compounds by Phanerochaete chrysosporium. Appl. Environ. Microbiol. 39, 535–540 (1980).
Harwood, C. S. & Parales, R. E. The beta-ketoadipate pathway and the biology of self-identity. Annu. Rev. Microbiol. 50, 553–590. https://doi.org/10.1146/annurev.micro.50.1.553 (1996).
Song, Y.-J. Characterization of aromatic hydrocarbon degrading bacteria isolated from pine litter. Korean J. Microbiol. Biotechnol. 37 (2009).
Peng, X., Misawa, N. & Harayama, S. Isolation and characterization of thermophilic bacilli degrading cinnamic, 4-coumaric, and ferulic acids. Appl. Environ. Microbiol. 69, 1417–1427. https://doi.org/10.1128/aem.69.3.1417-1427.2003 (2003).
Cao, B., Geng, A. & Loh, K.-C. Induction of ortho- and meta-cleavage pathways in Pseudomonas in biodegradation of high benzoate concentration: MS identification of catabolic enzymes. Appl. Microbiol. Biotechnol. 81, 99. https://doi.org/10.1007/s00253-008-1728-3 (2008).
Nakazawa, T. & Yokota, T. Benzoate metabolism in Pseudomonas putida(arvilla) mt-2:Demonstration of two benzoate pathways. J. Bacteriol. 115, 262–267. https://doi.org/10.1128/JB.115.1.262-267.1973 (1973).
Rajkumari, J., Paikhomba Singha, L. & Pandey, P. Genomic insights of aromatic hydrocarbon degrading Klebsiella pneumoniae AWD5 with plant growth promoting attributes: A paradigm of soil isolate with elements of biodegradation. 3 Biotech https://doi.org/10.1007/s13205-018-1134-1 (2018).
Kemp, M. B. & Hegeman, G. D. Genetic control of the beta-ketoadipate pathway in Pseudomonas aeruginosa. J. Bacteriol. 96, 1488–1499. https://doi.org/10.1128/JB.96.5.1488-1499.1968 (1968).
Medić, A. et al. A comprehensive study of conditions of the biodegradation of a plastic additive 2,6-di- tert -butylphenol and proteomic changes in the degrader Pseudomonas aeruginosa san ai. RSC Adv. 9, 23696–23710. https://doi.org/10.1039/C9RA04298A (2019).
Prado, R. et al. Lignin oxidation and depolymerisation in ionic liquids. Green Chem. 18, 834–841. https://doi.org/10.1039/C5GC01950H (2016).
Sainsbury, P. D. et al. Breaking down lignin to high-value chemicals: The conversion of lignocellulose to vanillin in a gene deletion mutant of Rhodococcus jostii RHA1. ACS Chem. Biol. 8, 2151–2156. https://doi.org/10.1021/cb400505a (2013).
Liu, W. et al. Whole genome analysis of halotolerant and alkalotolerant plant growth-promoting rhizobacterium Klebsiella sp. D5A. Sci. Rep. 6, 26710. https://doi.org/10.1038/srep26710 (2016).
Woo, H. L. et al. Complete genome sequence of the lignin-degrading bacterium Klebsiella sp. strain BRL6–2. Stand Genomic Sci. 9, 19–19. https://doi.org/10.1186/1944-3277-9-19 (2014).
Prabhakaran, M., Couger, M. B., Jackson, C. A., Weirick, T. & Fathepure, B. Z. Genome sequences of the lignin-degrading Pseudomonas sp. strain YS-1p and Rhizobium sp. strain YS-1r isolated from decaying wood. Genome Announc. 3, e00019-00015. https://doi.org/10.1128/genomeA.00019-15 (2015).
He, C., Li, Y., Huang, C., Chen, F. & Ma, Y. Genome sequence and metabolic analysis of a fluoranthene-degrading strain Pseudomonas aeruginosa DN1. Front. Microbiol. https://doi.org/10.3389/fmicb.2018.02595 (2018).
Acknowledgements
We sincerely thank Associate Professor Philip D. Round for revising the manuscript.
Funding
This was supported by Mahidol University and the Center of Excellence in Biodiversity (BDC), Office of Higher Education Commission, Bangkok, Thailand (BDC-PG1-160004).
Author information
Authors and Affiliations
Contributions
T.S: Writing—Original Draft, Sample collection, Supervision, Project administration. E.A: Methodology, Validation, Investigation, Writing—Original Draft & Editing. S.C: Conceptualization, Sample collection, Supervision, Writing—Review & Editing, Funding acquisition. T.P: FE-SEM sample preparation and analysis. W.L.: GPC sample preparation and phylogenetic tree construction. P.O: Sample collection, Writing—Review & Editing. P.H: Conceptualization, Sample collection, Validation, Supervision, Writing—Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sumranwanich, T., Amosu, E., Chankhamhaengdecha, S. et al. Evaluating lignin degradation under limited oxygen conditions by bacterial isolates from forest soil. Sci Rep 14, 13350 (2024). https://doi.org/10.1038/s41598-024-64237-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64237-8
- Springer Nature Limited