Abstract
The presence of lignin and its derivatives in pulp effluent increases the pollution load on the environment. With an aim of better degradation and decolourization of wastewater, this study proposes the utilization of different ligninolytic enzymes from novel bacterial strains. Four novel bacterial strains of Klebsiella pneumoniae (K. pneumoniae strains NITW715076, NITW715076_1, NITW715076_2 and NITW715076_3) were isolated and identified. The ligninolytic enzymes were characterized by plate assay method. For the optimization of various process parameters of effluent sample, different approaches were used like one factor at a time and statistical optimization through response surface methodology (RSM). Further to validate the above data, enzyme activity, total phenolic concentration, GC–MS analysis and seed germination test were also performed. The ligninolytic enzymes produced were characterized as laccase and Manganese peroxidase (MnP). In lignin degradation and decolourization studies, consortia 1 (K. pneumoniae NITW715076_2 + K. pneumoniae NITW715076_1) (82.31%) was found more effective when compared to axenic culture (K. pneumoniae NITW715076_2) (74.1%). In RSM studies, Laccase and MnP activities were increased by 20% and 18%, respectively, as compared to one factor at a time optimization method. In addition, the enzyme activity for laccase and MnP after prediction by RSM was found 53338 IU/L and 147900 IU/L, respectively. The R2 values for both the enzymes were found to be significant. Further, GC–MS analysis also showed the degradation of different organic pollutants in effluent. Lastly, the seed germination test using consortia 1 corroborated the evidence of detoxification of industrial effluent. Effluent treated by consortia 1 showed better results in degradation and decolourization of lignin and their derivatives. Therefore, consortia 1 can be used for various industrial purposes like fruit juice clarification, diagnostic purposes and enhanced bioethanol production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulp and paper industries utilize lignocellulosic components of plants and chemicals during the process of manufacturing and cause environmental pollution due to discharge of waste material into the environment. At the time of pulp preparation, huge amount of inorganic compounds are used which react with lignin-like compounds present in lignocellulose and forms toxic organic compounds in effluent. Lignin present in wood pulp causes paper to turn yellow, so its removal from wood pulp is a necessary step to improve the quality of paper used in pulp and paper mills. The effluent from paper industry is characterized by its dark brown colour, high temperature, strong odour, high alkaline pH, very high chemical oxygen demand (COD 200,000 mg/l) and biological oxygen demand (40,000–50,000 mg/l). Pulp and paper mill effluent contains large number of organic compounds such as polycyclic aromatic hydrocarbons (PAHs), chlorophenols, dioxins and furans. These compounds are toxic in nature and show carcinogenic and mutagenic effect on aquatic biota. Colour of black liquor is mainly due to the presence of lignin and its derivatives and polymerized tannins. These compounds are recalcitrant in nature because of the presence of carbon–carbon biphenyl linkage (Lindholm-Lehto et al. 2015; Mishra and Thakur 2010). Comprehensive treatment technology is required for decolourization and detoxification of pulp and paper mill effluent as it causes detrimental effects on human such as respiratory effects, oxidative stress, liver damage and genotoxicity (Hubbe et al. 2016).
Conventional treatment methods such as lignin precipitation by acids followed by burning are in use, but they are very costly, inefficient and produce huge amount of sludge which is difficult to handle. Moreover, landfilling is not possible if the amount of sludge produced is more and also produces volatile organic toxic compounds such as dioxins on burning (Kalia 2008). Generally, pulp and paper industries also use recovery boilers to recover and burn black liquor which is produced during delignification process of paper. Use of biological methods such as utilization of lignin-degrading microorganisms and their enzymes has become attractive because of their economical approach to treat black liquor and making it suitable for discharge in eco-friendly way, which migrates the negative impact on environment (Mishra and Thakur 2010).
Fungi such as white rot (Phanerochaete chrysosporium, Trametes versicolor, etc.), soft rot and brown rot (Fomitopsis palustris) have been reported as lignin degrader. In contrast, so much research on bacteria has been done to obtain the good evidence for existence of ligninolytic activity. Several bacterial strains (Bacillus cereus, Serratia marcescens, Streptomyces viridosporus T7A, Pseudomonas species, Bacillus species, etc.) have been reported for degradation of lignin and its derivatives (Lotfi 2014). Citrobacter freundii and Serratia marcescens were found to be effective in removing lignin from pulp and paper industry wastewater (Abhishek et al. 2017). The importance of ligninolytic bacteria rose because of the role of bacteria in degrading lignin in aquatic ecosystem as wood-degrading bacteria have wider tolerance of pH, temperature and oxygen limitation than fungi (Mishra and Thakur 2010). Studies have been reported, wherein bacteria utilizes lignin oligomers as a sole carbon source and energy that produces enzymes and cleave inter-monomeric linkages of lignin (Rinaldi et al. 2016).
MnP is a glycoprotein having high redox potential, contains heme group and requires H2O2 for its activity. It catalyses the peroxidase-dependent oxidation of Mn(II) to Mn(III) which results in the formation of manganese oxalate and thus released from the enzyme surface(Hammel 1995). These metal complexes act as a reactive redox mediator for the oxidation of phenolic lignin substructures and dimers. When H2O2 is not present, MnP uses these free radicals as a source of peroxidase and increases the lignin-degrading efficiency (Chandra and Singh 2012). While laccases are the glycosylated glycoprotein containing multi-copper groups and requires molecular oxygen for the oxidation of various aromatic compounds by redox reactions, laccase catalyses the electron reduction in dioxygen and breaks it into two water molecules to oxidize a wide variety of substrates such as phenols, anilines and thiols. Thus, these mediators have the ability to react with high redox potential substrate to form a non-enzymatic route of oxidative polymerizing or depolymerizing reactions (Chandra and Singh 2012).
It is essential to optimize the composition and condition of culture media for effective decolourization and detoxification of black liquor to reduce pollution load. In present study, different parameters like pH, temperature, agitation speed, inoculum volume, carbon source and nitrogen source were optimized using one factor at a time and final prediction was done using response surface methodology (RSM) to identify minimum number of experiment and impact of individual factor. Therefore, the objectives of this study were to isolate and identify bacteria from contaminated site and optimize all the parameters and established best possible culture conditions by correlating all the parameters. Furthermore, the study has extended to examine the ability of the mixed cultures [consortia 1 (K. pneumoniae NITW715076_2 + K. pneumoniae NITW715076_1) and consortia 2 (K. pneumoniae NITW715076_2 + K. pneumoniae NITW715076)] to decolourize lignin and phenolic compounds along with the reduction in the BOD and COD of the industrial wastewater. Moreover, the metabolite characterization by GC–MS and toxicity assessment by seed germination method were also studied for the safe disposal of effluent in environment.
Materials and methods
Sample collection, preparation of media, isolation and purification of potential bacterial strains from natural environment
The effluent sample was collected from Pulp and paper industry of Warangal district in India. This effluent contained significant amount of chlorophenols and lignin. Later, the effluent was stored at 4 °C and used for further physico-chemical analysis.
Lignin basic media (LBM) were used for the isolation of bacteria with the composition (g/L) of: KH2PO4—1, yeast extract—0.01, ammonium tartrate diabasic—0.5, CuSO4 · 5H2O—0.001, MgSO4 · 7H2O—0.5, ferrous sulphate—0.001, CaCl2 · 2H2O—0.01, MnSO4 · H2O—0.001, lignin—0.499 and was supplemented with 1.5% w/v agar. After autoclaving 0.1% (w/v), glucose was added and media were used for isolation of potential lignin-degrading bacteria by serial dilution method (Atri and Sharma 2012).
Screening and characterization of potential bacterial strains for ligninolytic activity
Plate assay method was used for screening of isolated and purified bacterial strains. Different substrates were used for screening of different enzymes. MnP-producing bacteria oxidize phenol red and gives yellow colour, while laccase-producing bacteria oxidize guaiacol which changes the media colour to brown (Renugadevi et al. 2011). For laccase [1.11.1.13], guaiacol (0.01%) was used as a substrate in nutrient agar medium. After 48 h of the bacterial growth on agar plates, 0.0012% of guaiacol was added on the bacterial colonies which turn the positive strains into brown colour. While in case of MnP, glucose peptone lignin (GPL) media was used and phenol red (0.001%) was used as an indicator. The composition of glucose peptone lignin media (GPL) (g/L) was: glucose—20, peptone—5, KH2PO4—1, MgSO4 · 7H2O—0.5, lignin (500 ppm)—0.49 and agar—15 and the positive bacterial colonies turned into yellowish colour (Kumar et al. 2011).
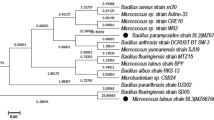
The bacterial cultures which showed ligninolytic activity were send to Macrogen Inc., Korea, for 16S rRNA sequence analysis and were deposited in NCBI. A phylogenetic tree was constructed using multiple sequence alignment which shows the close relationship between the strains.
Physico-chemical analysis of effluent before and after bacterial treatment
The effluent sample collected from pulp and paper industry was analysed for physico-chemical parameters before and after treatment of wastewater as per the standard methods for wastewater analysis. Physico-chemical parameters, i.e. pH was measured by using electrometric method, BOD by titrimetric method, COD by open reflux method, total solids, total nitrogen by Macro Kjeldahl method and total phenol by Folin–Ciocalteu method (Federation and Association 2005; Kamtekar et al. 2014). Heavy metal analysis was done by inductively coupled plasma atomic emission spectroscopy (ICP-AES), and lignin estimation was done according to Pearl and Benson (K 1990).
Process parameter optimization of potential bacterial isolates by one factor at a time
For process parameter optimization, the real wastewater sample was mixed with synthetic media in 1:1 ratio (modified media). Single parameter optimization was done to assess the consequence of each parameters on enzyme activity: pH (5.5–9.5), temperature (30, 35, 37, 40 and 45 °C), agitation speed (120–150 rpm), inoculum size (1–7 ml), carbon source (1% w/v) (glucose, lactose, maltose, fructose, mannose and sucrose) and nitrogen sources (0.5% w/v) (ammonium sulphate, peptone, yeast extract and urea) (Hariharan and Nambisan 2012). Bacterial isolates which gave the optimum laccase and MnP production in plate assay method were selected here for optimization process.
Parameter optimization and prediction using RSM
After process parameter optimization of potential bacterial isolates by one factor at a time, the optimization of modified media was carried out by RSM. The defined preferred response was laccase and MnP activity as a measure of lignin and phenol degradation by potential bacterial isolates during degradation. A three-factor central composite design well appropriate for process optimization was amended with optimum pH, temperature, inoculum volume, agitation speed, 1% carbon source and 0.5% nitrogen source obtained after one factor at a time optimization process. Duration of degradation (A), wastewater% (B) and inducer dose (C) were taken as independent variables, at five levels (− 2, − 1, 0, + 1 and + 2). The mutual interaction between the independent variables was studied by using CCD to develop a mathematical correlation amongst selected parameters and laccase and MnP activity. In this method, data analysis, experimental design, quadratic model building and response surface were plotted using the statistical software package Design Expert® 10.0 (State- Ease, Inc., Minneapolis, MN, USA). All the experiments were conducted in a set of three, and their mean was recorded as a response. Numerical optimization was implemented for validating the recorded response against the predicted one by setting the response to maximum when all parametric values were set “in range”. The detailed statistical parameters and design matrix generated after experimental optimization are mentioned in Tables 1 and 2 (Diwan et al. 2018; Ravikumar et al. 2005).
Degradation of pulp and paper mill effluent by axenic and mixed culture
After optimization process, the degradation studies were done by using axenic and mixed culture. The optimum value of each parameter was used to get the maximum enzyme production for degradation of lignin and phenolic compounds. In axenic culture, optimized single strain of Klebsiella pneumonia was used, while in mixed culture consortia 1 and consortia 2 were prepared for degradation studies. Bacterial growth, ligninolytic enzyme assay, total phenol and lignin estimation were checked. The mixed culture in this experiment was prepared to check the combined effect of different strains of Klebsiella pneumoniae grown in same flask in degradation of lignin and phenolic compounds along with the enhanced laccase and MnP activity as compared to the axenic culture.
Bacterial growth
Bacterial biomass was measured in terms of increase in culture absorbance at 620 nm with spectrophotometer (Jasco V-630).
Ligninolytic enzyme assay
The well-grown bacterial cultures were inoculated into LBM broth for the production of laccase and manganese peroxidase enzymes and incubated at 37 °C for 54 h. In case of axenic and mixed culture, bacterial culture(s) were inoculated into modified media for enzyme production and incubated at optimum conditions for 216 h. The amount of enzymes produced was assayed at regular time intervals using spectrophotometer.
Activity of laccase was measured with the oxidation of 2,2-azino-bis (3-ethylbenzthiazoline)-6-sulphonate (ABTS) at 25 °C. The reaction mixture contained 300 µl of 0.1 M citrate buffer (pH-5), 0.1 ml of 0.3 mM ABTS (ε420 = 3.6 × 104 M−1 cm−1) and 0.6 ml of culture filtrate in total volume of 1 ml. The reaction was monitored by increase in absorbance at 420 nm (Bourbonnais et al. 1997). The enzyme activity is expressed as U/L of substrate oxidized per litre per minute, in the following equation:\({\text{UL}}^{ - 1} \,\min^{ - 1} = \frac{{\Delta {\text{Abs}} \times V}}{v \times \varepsilon \times T}\), where ΔAbs is the absorbance change in per minute at particular wavelength, ε = extinction coefficient of each substrate at particular wavelength (mol−1 cm−1), V = final volume of the reaction mixture in millilitre, and T = reaction time in minute (Desai et al. 2011).
Manganese-dependent activity was determined by measuring the increase in Mn+3 malonate formation at 610 nm (ε610 = 4460 M−1 cm−1) at 25 °C. The assay mixture contained 0.5 ml of 0.1 M citrate buffer (pH-5), culture filtrate-250, 50 µl of 0.1% phenol red, 100 µl of 250 mM sodium lactate, 25 µl of 2 mM manganese sulphate, 100 µl of 0.5% bovine serum albumin and 25 µl of 0.2 mM H2O2. The reaction was monitored by increase in absorbance at 610 nm. The enzyme activity is expressed as U/L of substrate oxidized per litre per minute and was calculated by the above-mentioned equation which used to calculate laccase activity (Vares et al. 1995).
Total phenol and lignin estimation
Phenol estimation was done by Folin–Ciocalteu method (Oliveira et al. 2009). In this method, the mixture contained 1 ml of enzyme, 5 ml of distilled water (D/W) and 500 µl of Folin–Ciocalteu reagent was incubated at room temperature for 5 min. After incubation, 1.5 g of 20% sodium carbonate was added and was made up to 10 ml with distilled water. After 2 h of incubation, blue colour was developed and absorbance was taken at 750 nm (Kamtekar et al. 2014).
Lignin estimation in supernatant was checked by adjusting its pH to 7.0 with 2 M NaOH. Fifty ml of sample was mixed with 1 ml of 10% CH3COOH and NaNO2. Two ml of NH4OH was added after 15 min, and the absorbance was checked at 430 nm after 5 min of incubation. In addition, for blank, 50 ml of distilled water was mixed with 1 ml CH3COOH (10%) and 2 ml of NH4OH. One ml of NaNO2 (10%) was added after 15 min, and the absorbance was checked at 430 nm after 5 min of incubation (Sasidharan et al. 2015). The obtained absorbance values were converted into lignin content (ppm) by using following formula:
GC–MS analysis for metabolite characterization
Gas chromatography–mass spectroscopy (GC–MS) analysis was done at SAIF, IIT Bombay, India, for the characterization of different metabolic products before and after bacterial treatment. The treated and untreated bacterial samples were centrifuged at 8000 rpm for 15 min. The supernatant was extracted with equal volume of dichloromethane (DCM) and dried. The dried residue was derivatized with trimethylsilyl (TMS). In this method, 100 µl pyridine methoxy hydroxylamine (1 mg/ml) and 100 µl trimethylsilyl [BSTFA (N, O-bis trimethylsilyl) trifluoroacetamide and TMCS (trimethylchlorosilane)] were added. The mixture was heated at 65 °C for 1 h with periodic shaking to dissolve residues, and 4 µl of silylated compounds was injected in GC–MS system. The helium gas was used as a carrier gas with flow rate of 1 ml/min, and the column temperature programme was 50 °C (5 min) and 50–280 °C (10 °C/min, hold time 7 min). The transfer line and ion source temperature were maintained at 200 and 250 °C. The pollutants present in sample before and after treatment were identified by comparing the mass spectra with National Institute of Standard Technology (NIST) database library software available with instrument (Haq et al. 2016).
Seed germination test for toxicity assessment
In this experiment, different dilutions (10, 20, 40, 60, 80 and 100% v/v) of bacterial treated and untreated effluent were prepared and distilled water was used as a control. 5–6 seeds of Cicer arietinum were placed in different Petri-dishes lined with 2–3 layers of Whatman No. 1 filter paper (Whatman, UK) soaked with different dilutions of effluent samples. Petri-dishes containing the seeds of Cicer arietinum were placed in good moisture condition which requires temperature between 21 and 29 °C. The length of crop usually varies depending on the available moisture, heat and seed genotype, but it is usually in the range of 95–100 days (Kulkarni and Chimmad 2014). Periodic monitoring of seeds was done till 4 weeks to assess the per cent of germination (Gaur et al. 2017). All the experiments were done in triplicates. The germination rate (GI) was calculated by using Eq. (1), where GSS and GSC are the number of germinated seeds in the sample and control, respectively.
Results and discussion
Isolation, screening and characterization of bacterial strains
Microorganisms present in polluted environment have the capability to degrade recalcitrant pollutants without addition of chemicals to the system. In past few decades, Bacillus cereus, Serratia marcescens, Streptomyces viridosporus T7A, Pseudomonas species, Bacillus species, etc., have been reported for degradation of lignin. Though many fungi have been isolated which showed higher production of the ligninolytic enzymes, fungus requires solid media and cannot withstand extreme pH and temperature conditions.
Therefore, this work completely focused on bacterial isolates as it does not require solid medium and can withstand extreme pH and temperature conditions(de Gonzalo et al. 2016). Out of 25 bacterial strains which were isolated from effluent sample, four strains have displayed the positive response for the degradation of ligninolytic enzymes. On the basis of their 16S rRNA gene sequencing, the four bacterial strains were identified as NITW1 (Klebsiella pneumoniae strain NITW715076_2 Accession ID MF185143), NITW2 (Klebsiella pneumoniae strain NITW715076_1 Accession ID MF086672), NITW3 (Klebsiella pneumoniae strain NITW715076_3 Accession ID MF086677) and NITW4 (Klebsiella pneumoniae strain NITW715076 Accession ID KY435679) and submitted in NCBI. Accordingly, phylogenetic analysis was done using multiple sequence analysis tool and the results are shown in Fig. 1.
Physico-chemical characteristic of pulp and paper mill effluent before and after treatment
The pulp and paper mill effluent showed the higher amount of COD, BOD, TDS, TSS, lignin, total phenols and heavy metals before the bacterial treatment as mentioned in Table 3. After 216 h of incubation period, there was a significant reduction in COD, BOD, TDS, TSS, lignin, total phenols and heavy metals after bacterial treatment with axenic and mixed culture. Both axenic and mixed cultures favoured acidic condition (pH = 6.5 ± 0.43) for degradation of pollutants which indicates the formation of acidic compound through citric acid cycle by utilizing the simpler carbon compounds present in the modified media. Consortia 1 showed the better decolourisation and reduction in various parameters in effluent sample. The formation of acidic products during the lignin degradation in the media has been reported in earlier studies also (Chandra et al. 2011; Chandra and Singh 2012; Yadav and Chandra 2015). The axenic culture was able to reduce 49.9% COD, 34.5% BOD, 74.1% lignin and 61.9% total phenols after 216 h incubation, while consortia 1 and consortia 2 were proficient in reducing 89.9, 77.7% COD, 67, 55.4% BOD, 82.3, 68.98% lignin and 69, 62.3% total phenols, respectively, after 216 h incubation.
Process parameter optimization of potential bacterial isolates
Out of four bacterial strains Klebsiella pneumoniae strain NITW715076_2 exhibited higher laccase and MnP production as well as reduction in total phenols and lignin with synthetic media. Therefore, it was selected for optimization of different process parameters like temperature, pH, agitation speed, inoculum volume, carbon and nitrogen source with modified media.
To evaluate the effect of temperature, pH, agitation speed and inoculum volume on ligninolytic enzyme production by K. pneumoniae strain NITW715076_2, temperature range from 30 to 45 °C, pH range from 5.5 to 9.5, agitation range from 120 to 150 rpm, inoculum volume range from 1 to 7 ml (OD-0.6) and for different nutrient sources like carbon (glucose, fructose, lactose, mannose, maltose and sucrose (1% w/v)) and nitrogen (ammonium sulphate, yeast extract, urea and peptone at (0.5% w/v)) were chosen. The flasks were incubated for 216 h, and maximum ligninolytic activity at different optimized parameters was observed, at 35 °C (Lac 33611 IU/L and MnP 42870 IU/L), at pH 6.5 (Lac 31185 IU/L and MnP 36232 IU/L), at 130 rpm (Lac 39231 IU/L and MnP 52915 IU/L), for 4 ml inoculum volume (Lac 43569 IU/L and MnP 68296 IU/L), for 1% sucrose(Lac 43561 IU/L and MnP 77892 IU/L) and for 0.5% yeast extract (Lac 44546 IU/L and MnP 122152 IU/L) as shown in Fig. 2a–f, respectively. In present investigation, there was a gradual increase in laccase and MnP activity from 30 to 35 °C and at 37 °C the enzyme activity decreases. Increase in temperature from the optimal value leads to decrease in laccase and MnP activity due to increase in thermal instability. pH plays an important role for the growth, morphology of microorganism and may alter the 3D structure of enzyme upon changing the pH. The optimum pH for both the enzyme in this study was 6.5, after which both enzymes lost its activity. This may be attributed to the change in hydrogen ion concentration present in the medium. It has been reported that the carbon source is an important factor in laccase and MnP production. Since enzymes are substrate specific, media containing sucrose displayed the highest laccase and MnP activity. Although sucrose is a disaccharide which first will break down into glucose and fructose, it can easily be utilized by the microorganisms. It has already been discussed that higher amount of laccase and MnP activity by the organism is due to efficient and rapid utilization of substrate. Organic nitrogen source gives high laccase and MnP yield so medium containing continuous yeast extract was found to have highest laccase and MnP activity as enzymes are substrate specific. Yeast extract is a rich source of protein, amino acid, vitamins for microbial growth.
Chandra et al. (2007) reported maximum decolourization of lignin from Paenibacillus sp. Aneurinibacillus aneurinilyticus, Bacillus sp. at pH 7.6 and temperature 30 °C. In another finding, Citrobacter freundii and Serratia marcescens showed the maximum decolourization at pH 8.2, temperature 34 °C and agitation speed 140 rpm (Abhishek et al. 2017). Mishra and Thakur did similar work with Bacillus sp., and they observed the optimum decolourization and detoxification of pulp and paper mill effluent at pH 8.0, temperature 35 °C, agitation speed 200 rpm, sucrose-2.5% w/v, inoculum size 5%(w/v) and 10% black liquor (Mishra and Thakur 2010). From the literature review, it is confirmed that the optimum conditions such as pH, temperature, agitation speed, inoculum volume, carbon source and nitrogen source for lignin degradation and ligninolytic enzyme production in effluent differ greatly from one bacterial strain to another. The reason behind this may be different environmental conditions and utilization of different nutrients for its metabolic activity. Additionally, for degradation of lignin and its derivatives, bacteria produce different enzymes such as laccase and MnP. The results of this study revealed that at these optimum conditions, strains of Klebsiella pneumoniae are capable of degrading lignin and phenolic compounds.
Peter et al. optimized the culture condition using bacterial consortia of Pseudomonas aeruginosa and Pseudomonas fluorescens to get the maximum laccase activity. Laccase activity was optimum at temperature 40° C (Lac 0.0388 U/ml), pH 7.0 (0.0341 U/ml), 2% glucose (0.051 U/ml) and 2% peptone (0.038 U/ml) (Peter et al. 2014). MnP from bacteria has not been much explored, but few years ago Verma and Madamwar studied this enzyme from Serratia marcescens and found 55.3 U/ml MnP activity and 8.3 U/ml laccase activity at pH 7.0 and temperature 26 °C contrasting to our observations (Verma and Madamwar 2003). Findings of our study are competent and even better/higher than the contemporary reports.
Model development, regression analysis and optimization through RSM
In one parameter at a time optimization method, the maximum laccase (44.54 U/ml) and MnP (122.152 U/ml) activity were observed at pH 6.5, temperature 30 °C, inoculum volume 4 ml, agitation speed 130 rpm, carbon source 1% sucrose and nitrogen source 0.5% yeast extract. To further enhance the laccase and MnP activity by Klebsiella pneumoniae strain NITW715076_2, the process was optimized by RSM using CCD. The responses that were observed from the experimental runs were analysed with respect to analysis of variance (ANOVA) and regression coefficient. Thus, a quadratic polynomial regression equation (Eqs. 2 and 3) for laccase and MnP activity was obtained, showing variable interaction profile and their effect on preferred response.
The laccase and MnP activity predicted by Eqs. (2) and (3) is mentioned in Table 2 with the experimental results. The prediction of experimental data is statistical significant as the values calculated with the predictive equations are determined to be very close to the actual values (Fig. 3). ANOVA was used to evaluate the significance and adequacy of the model. ANOVA of regression parameters of the predicted response surface quadratic model for laccase and MnP activity using the results of experiments performed is given in Tables 4 and 5, respectively. Corresponding term will be more significant if the F value will be higher. In addition, the p value associated with the f value could be used to show whether the f value is large enough or not. If the probability > F less than 0.050, it indicates that the model terms are significant, while the values greater than 0.1 implies that the model terms are nonsignificant.
ANOVA details in Tables 4 and 5 validate the model equation and show that the CCD model has good fit. F values of regressions were found to be large enough and implies that the model is significant. Moreover, it can be seen from Tables 4 and 5 that the model is highly significant as the probability > F values of models were determined to be less than 0.0001 for the second-order polynomial. In case of laccase activity, model F value is 33.81 with corresponding p value of 0.0001 which indicates that the model is highly significant (Table 4) and can properly explain the relationship between factors and response. However, model F value is 222.65 with corresponding p value of 0.0001, implies that the A and B are significant model terms in case of MnP (Table 5). In case of laccase, interacting coefficients AB, AC and BC have significant effect on laccase activity, while they are showing nonsignificant effect on MnP activity. Finally, it can be concluded that R2 values in both the cases are desirable, i.e. R 2laccase = 0.9396 and R 2MnP = 0.9766, because of a good fit of the model.
After a total run of 20 sets of different experiments, the mutual interaction between variables was analysed by 3D surface and 2D contour plots. In addition, 2D plots of actual and predicted values of laccase and MnP activity were analysed (Fig. 3b, d). The response surface plots of laccase and MnP are illustrated in Fig. 3a and c, respectively, which indicates that the optimum operating conditions have maximum value of responses and are attributed to all variables in the design space. All the three factors showed enough interaction, but duration and wastewater% were significant than inducer dose in case of both the enzyme. The conditions determined by RSM study, adequate for maximum enzyme production, were 1 mg/ml inducer dose, 30% wastewater and 5 days of duration yielding 53338 U/L laccase activity while for Mnp 0.5 mg/l inducer dose, 30% wastewater and 8 days of duration yielding 143950 U/L MnP activity. The obtained laccase and MnP activity was sufficiently close to predicted values indicating the desirability of the model. The laccase and MnP activity was 44546 IU/L and 122152 IU/L, respectively, while after optimization with RSM its activity was increased by 20 and 18%, respectively.
Periodic monitoring of modified media degradation by axenic and mixed culture
Klebsiella pneumoniae strain NITW715076_2 has been shown to degrade lignin (74.16%) and phenols (61.91%) in LBM as well as in modified media (effluent: synthetic media (1:1 ratio)) at pH 6.5 containing 10000 ppm of lignin and 158.18 µg/ml of total phenols in 216 h incubation time. Mixed cultures (1:1) (consortia 1 and consortia 2) were also prepared to improve the degradation process. Consortia 1 was prepared by mixing K. pneumoniae strain NITW715076_2 and NITW715076_1, while consortia 2 was prepared by mixing K. pneumoniae strain NITW715076_2 and NITW715076. The bacterial growth in modified media was observed to be higher for both axenic and mixed cultures at 48 h incubation period followed by gradual increase up to 120 h and started decreasing till 216 h (Fig. 4). In mixed condition (consortia 1), the growth rate, decolourization of lignin and phenol reduction were high as compared to axenic culture. These finding showed that the bacterial consortia grow well in modified media and have enhancing effect on growth, lignin degradation and phenol reduction. These results are very similar with the observations of other studies (Singh et al. 2008; Yadav and Chandra 2015).
The degradation of lignin and phenol reduction at initial phase was low in spite of fast bacterial growth which might be due to utilization of simpler form of carbon (sucrose) and nitrogen (yeast extract) available in the modified media. But as the starvation period starts bacteria will start utilizing lignin as a co-substrate. This co-metabolism mechanism has been reported by many researchers in bacteria and fungi for degradation of lignin containing effluent (Asina et al. 2016; Xu et al. 2018; Yadav and Chandra 2015). During degradation process, reduction in lignin (up to 2583.34 ppm (Figs. 4, 5)) and total phenols (up to 60.24 µg/ml (Figs. 4, 5)) were also recorded, by axenic culture. However, consortia 1 reduced lignin and total phenols up to 1769.23 ppm and 50.66 µg/ml, respectively (Figs. 4, 5). Axenic culture of K. pneumoniae strain NITW715076_2 was found to degrade lignin 74.1% at optimized conditions (35 °C, pH 6.5, 130 rpm, 216 h, 1% sucrose and 0.5% yeast extract). In addition, consortia 1 and consortia 2 reduced the lignin content by 82.3 and 68.98%. Yadav and Chandra did similar work by preparing syntrophic co-culture of Bacillus subtilis and Klebsiella pneumonia for degradation of lignin, and they found that the combined effect of these two bacteria was able to degrade only 58% of lignin (Yadav and Chandra 2015). Xu et al. reported lignin degradation by Klebsiella pneumoniae NX-1, Pseudomonas putida NX-1 and Ochrobactrum tritici NX-1 25, 30 and 20%, respectively (Xu et al. 2018). Similarly, Bacillus sp. strains CS-1&CS-2 removed 61% of lignin (Chang et al. 2014). In another finding that corroborated to this work, 50% and 55% of lignin were degraded by Citrobacter freundii and Serratia marcescens, respectively. However, the mixed culture of same bacteria showed 70% lignin degradation (Abhishek et al. 2017). From these results, it could be inferred that the results of our study are proficient and even better than the existing reports.
At similar optimum condition, axenic culture of Klebsiella pneumoniae strain NITW715076_2 was found to degrade 61.9% phenol, while consortia 1 and consortia 2 reduced the phenol content to 69% and 62.3%, respectively. Initially, the phenol content increased due to the breakdown of lignin into phenolic compounds, later it was utilized by bacteria as a carbon source. Chandra and Abhishek observed the 71.42% reduction in total phenol by Citrobacter freundii (FJ581022) and Citrobacter sp. (FJ581023) (Chandra and Abhishek 2011). In another study, Pseudochrobactrum glaciale (FJ581024), Providencia rettgeri (GU193984) and Pantoea sp. (FJ755943) degraded phenol by 84.8, 82 and 75%, while mixed culture of these bacteria degraded phenol by 86.2%. The phenol degradation results of this research showed decreased degradation as compared to reported studies, but the efficiency of these strains could be improved by co-culturing it with another bacterial strain (Chandra and Singh 2012).
The results presented in Table 3 showed that, axenic culture significantly reduced BOD (35%) and COD (50%). However, maximum reduction was observed when modified media were treated with mixed culture (COD 90% and BOD 67%). The reduction in total phenols and COD during this study can be considered as the result of lignin and organic compounds degradation. Mixed culture of Bacillus subtilis and Klebsiella pneumonia reduced the BOD and COD up to 62 and 73% respectively (Yadav and Chandra 2015). In another study, 67% of COD reduction was observed by mixed bacterial cultures of Citrobacter freundii and Serratia marcescens (Abhishek et al. 2017). Neoh and his co-worker bio-remediated the high strength agricultural wastewater using Ochrobactrum sp. strain SZ1 and reduced the COD by 71%. In contrast to the present results, Singh et al. reported 98 and 99% reduction of BOD and COD by mixed culture of Bacillus sp. (AY952465) and S. marcescens (DQ002385) after 168 h of degradation (Singh et al. 2008).
During lignin degradation, laccase, MnP and lignin peroxidase (LiP) are generally secreted by microorganisms. LiP activity was not seen in this work as it takes longer duration for its secretion. Higher laccase (44546 IU/L) and MnP (122152 IU/L) activity was noted at 216 h of incubation in case of axenic culture. The degradation process of ligninolytic enzymes could be improved by mixed culture as compared to axenic culture (Fig. 4). In case of mixed culture, maximum enzyme activity was recorded at 216 h of incubation (Laccase 49556 IU/L and MnP 134619 IU/L). Production of ligninolytic enzymes during lignin degradation showed direct correlation and were identified and characterized in many bacteria and fungi by various researchers, but the proper documentation of these enzymes for lignin degradation as well as decolourization has not been reported (Singh et al. 2008; Yadav and Chandra 2015). Existence of these enzymes in single bacterial strains is a rare property, and they work mutualistically in the medium (Janusz et al. 2013). Laccase and MnP have a broad range of substrate specificity due to their high redox potential. These two enzymes are ligninolytic enzymes which are involved in the degradation of both phenolic and non-phenolic pollutants present in the environment. Laccase and MnP activity from co-culture of Bacillus subtilis (GU193980) and Klebsiella pneumoniae (GU193981) was 4.5 and 2.3 U/ml (Yadav and Chandra 2015). In another finding, P. putita NX-1, O. tritici NX-1 and K. pneumoniae NX-1 were used for ligninolytic enzyme activity study. Out of three isolates, only P. putita NX-1 showed the maximum laccase (635.9 U/L), LiP (6497.2) and MnP activity (599.7 U/L) (Xu et al. 2018). Chang et al. reported the laccase activity by Bacillus sp. strain CS-1 (3.5 U/L) and CS-2 (3.0 U/L). These strains showed very low MnP activity (data not shown), while LiP activity was not observed (Chang et al. 2014). In contrast to the results obtained in present study, Lai and co-worker worked on thermophilic lignin-degrading bacteria and reported that out of ten isolates CLMT 18 (0.7475 U/ml) and CLMT 34 (0.8178 U/ml) showed the maximum laccase activity, while CLMT5 (1.8759 U/ml), CLMT 18 (1.6684 U/ml) and CLMT 20 (1.5322 U/ml) showed maximum MnP activity (Lai et al. 2017).
Metabolite characterization
Most researchers emphasize only on optimization of the process, and very few studies are carried out which focuses on compositional analysis during the biodegradation process. However, in the present study, degradation of lignin, their derivatives and phenolic compounds were detected before and after treatment by GC–MS analysis. Peaks detected in control sample disappeared after degradation and appearance of new peaks indicated the generation of new metabolites. The detail study of the compounds generated during degradation process is listed in Table 6.
The compounds present in the treated and untreated effluent were extracted with dichloromethane and are shown in Fig. 6. The number of peaks in bacterial degraded sample is reduced in total ion chromatogram as compared to control. Peaks detected in control at different retention time (RT) were 2-chlorophenol (RT3.76), 2,4,6-trichlorophenol (RT6.76), propanoic acid (RT8.12), pentachlorophenol (RT11.15), 3-cyclohexane 1-methanol (RT12.31), 2-methoxy phenol (14.39), 1,2-butanediol (RT14.48), benzoic acid (RT15.05), 2-methoxy propanoyl chloride (RT 18.90), 4-hydroxy-3,5-dimethoxy benzaldehyde (RT19.12), tetradecanoic acid (RT 21.05), hexanedioic acid (RT21.21), dibutyl phthalate (RT21.55), benzene acetic acid (RT22.24), 1-chloro-heptacosane (RT23.14), oxalic acid, heptyl propyl ester (RT24.86). Benzoic acid (RT15.05), tetradecanoic acid (RT20.49), hexanedioic acid (RT21.21) and benzene acetic acid (RT22.24) were also present in the untreated effluent and was also reported in another findings (Chandra et al. 2011). Low molecular weight compounds such as 2-chlorophenol (RT3.76), 2,4,6-trichlorophenol (RT6.76) and propanoic acid (RT8.12) were formed due to the degradation of long-chain alkane compounds, whereas high molecular weight compounds break into low molecular simple compound and small phenolic subunits during degradation process by aromatic ring cleavage. The compounds detected in pulp mill effluent are known to be endocrine disrupting compounds (Chandra and Singh 2012; Yadav and Chandra 2015). In this study, the results of GC–MS analysis confirmed the degradation or transformation of compounds into simpler one which results in the change in effluent from dark brown colour to pale yellow and after that no colour reduction was observed. Therefore, there is a need of further treatment of treated effluent for complete decolourisation.
Seed germination test for toxicity assessment
Seed germination is a complicated physiological and biochemical process in plants that can get affected extremely through a lot of environmental factors. The primary component of most of the world’s crop is starch, and degradation of starch is crucial for seed germination. Starch degradation is initiated by α-amylase in the process of seed germination, producing soluble oligosaccharides from starch. After that, these are hydrolysed by α-amylase to release maltose, and lastly, maltose is broken down by glucosidase to form glucose providing energy to the germinating seeds. Untreated effluent and tap water were the two controls that were used. Untreated effluent was used as a control to compare the effectiveness of the treatment in reducing toxicity.
Seed germination test revealed that the untreated effluent above 20% (v/v) inhibited germination of seed, while in treated effluent seed germination was seen up to 60% (v/v). The reason might be due to the presence of optimum level of organic nutrients essential for plant growth. In addition, the reduction in seed germination might be due to higher load of pollutant content affecting various physiological and biochemical processes during seed germination. Moreover in untreated effluent, the germination rate was decreased after 20%, and this might be due to reduction in amylase activity, but in case of treated effluent, the amylase activity was reduced after 60% (Table 7) (Chandra et al. 2011).
Conclusion
Lignin is a polymeric compound which is abundantly present in pulp, and paper mill effluent results in the formation of various phenolic compounds that have adverse effect on ecosystem. So, it is necessary to find an enzymatic route for the complete degradation of such phenolic compounds into biodegradable compounds. The ligninolytic enzyme is currently considered an ideal green catalyst. They have several applications in different fields like food, textile, pulp and paper, pharma, cosmetic, paint or furniture industries. They can also be used as a biosensor for therapeutic use and also can be used for fruit juice clarification. Therefore, there is a need for the enhanced protein production of these enzymes for the betterment of the ecosystem.
This study revealed that the Klebsiella pneumoniae strain NITW715076_2 was capable of 74.5% decolourization of effluent from pulp and paper industry at optimized condition. In addition, the GC–MS analysis of the control and bacterial degraded sample showed that the bacteria utilize the phenolic compounds rather than their biotransformation. Hence, the developed bacterial consortia 1 was capable of effective decolourization (82.3%) and detoxification (80%) of pulp and paper effluent for environmental safety. The optimization of process parameters for decolourization and detoxification was studied with one factor at a time and RSM. The laccase and MnP activity was increased at optimum value of pH: 6.5, temperature: 35 °C, agitation speed: 130 rpm, inoculum size: 4 ml, carbon source (1%): sucrose and nitrogen source (0.5%): yeast extract. The parameters were studied at various time intervals: 5–8 days, wastewater%: 30–50 (v/v) and inducer dose: 0.5–1 mg/L. The optimized values of parameters found using RSM were 5 days of duration, wastewater% 30 v/v and inducer dose 1 mg/l in case of laccase, while for MnP the optimized values of parameters were duration 6.5 days, wastewater 23.182 v/v and inducer dose 0.75 mg/L.
References
Abhishek A, Dwivedi A, Tandan N, Kumar U (2017) Comparative bacterial degradation and detoxification of model and kraft lignin from pulp paper wastewater and its metabolites. Appl Water Sci 7:757–767. https://doi.org/10.1007/s13201-015-0288-9
Asina F, Brzonova I, Voeller K, Kozliak E, Kubátová A, Yao B, Ji Y (2016) Biodegradation of lignin by fungi, bacteria and laccases. Bioresour Technol 220:414–424. https://doi.org/10.1016/j.biortech.2016.08.016
Atri NS, Sharma SK (2011) Qualitative estimation of cellulases and lignin modifying enzymes in five wild lentinus species selected from North West India. Acad J Plant Sci 4(4):105–109
Bourbonnais R, Paice M, Freiermuth B, Bodie E, Borneman S (1997) Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl Environ Microbiol 63:4627–4632
Chandra R, Abhishek A (2011) Bacterial decolorization of black liquor in axenic and mixed condition and characterization of metabolites. Biodegradation 22:603–611
Chandra R, Singh R (2012) Decolourisation and detoxification of rayon grade pulp paper mill effluent by mixed bacterial culture isolated from pulp paper mill effluent polluted site. Biochem Eng J 61:49–58
Chandra R, Raj A, Purohit H, Kapley A (2007) Characterisation and optimisation of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemosphere 67:839–846
Chandra R, Abhishek A, Sankhwar M (2011) Bacterial decolorization and detoxification of black liquor from rayon grade pulp manufacturing paper industry and detection of their metabolic products. Bioresour Technol 102:6429–6436
Chang Y-C, Choi D, Takamizawa K, Kikuchi S (2014) Isolation of Bacillus sp. strains capable of decomposing alkali lignin and their application in combination with lactic acid bacteria for enhancing cellulase performance. Bioresour Technol 152:429–436. https://doi.org/10.1016/j.biortech.2013.11.032
de Gonzalo G, Colpa DI, Habib MHM, Fraaije MW (2016) Bacterial enzymes involved in lignin degradation. J Biotechnol 236:110–119. https://doi.org/10.1016/j.jbiotec.2016.08.011
Desai SS, Tennali GB, Channur N, Anup A, Deshpande G, Murtuza BA (2011) Isolation of laccase producing fungi and partial characterization of laccase. Biotechnol Bioinf Bioeng 1:543–549
Diwan B, Parkhey P, Gupta P (2018) Platform study on the development of a nondetoxified rice straw hydrolysate to its application in lipid production from mortierella alpina. ACS Sustain Chem Eng 6:1225–1234. https://doi.org/10.1021/acssuschemeng.7b03530
Federation WE, Association APH (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington
Gaur N, Kukreja A, Yadav M, Tiwari A (2017) Assessment of phytoremediation ability of Coriander sativum for soil and water co-contaminated with lead and arsenic: a small-scale study 3. Biotech 7:196. https://doi.org/10.1007/s13205-017-0794-6
Hammel KE (1995) Mechanisms for polycyclic aromatic hydrocarbon degradation by ligninolytic fungi. Environ Health Perspect 103:41
Haq I, Kumar S, Kumari V, Singh SK, Raj A (2016) Evaluation of bioremediation potentiality of ligninolytic Serratia liquefaciens for detoxification of pulp and paper mill effluent. J Hazard Mater 305:190–199. https://doi.org/10.1016/j.jhazmat.2015.11.046
Hariharan S, Nambisan P (2012) Optimization of lignin peroxidase, manganese peroxidase, and lac production from Ganoderma lucidum under solid state fermentation of pineapple leaf. BioResources 8:250–271
Hubbe MA et al (2016) Wastewater treatment and reclamation: a review of pulp and paper industry practices and opportunities. BioResources 11:7953–8091
Janusz G, Kucharzyk KH, Pawlik A, Staszczak M, Paszczynski AJ (2013) Fungal laccase, manganese peroxidase and lignin peroxidase: gene expression and regulation. Enzyme Microbial Technol 52:1–12
Kalia VC (2008) Microbial treatment of domestic and industrial wastes for bioenergy production. In: Applied microbiology, NISCAIR, CSIR, New Delhi
Kamtekar S, Keer V, Patil V (2014) Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed polyherbal formulation
Kulkarni M, Chimmad V (2014) Effect of temperature regimes on phenology and yield of chickpea (Cicer arietinum L.). Karnataka J Agric Sci 27:526–527
Kumar VV, Kirupha SD, Periyaraman P, Sivanesan S (2011) Screening and induction of laccase activity in fungal species and its application in dye decolorization. Afr J Microbiol Res 5:1261–1267
Lai C, Chua H, Danquah M, Saptoro A (2017) Isolation of thermophilic lignin degrading bacteria from oil-palm empty fruit bunch (EFB) compost. In: IOP conference series: materials science and engineering, 2017. vol 1. IOP Publishing, p 012016
Lindholm-Lehto PC, Knuutinen JS, Heidi S, Ahkola J, Herve SH (2015) Refractory organic pollutants and toxicity in pulp and paper mill wastewaters. Environ Sci Pollut Res Int 22:6473
Lotfi G (2014) Lignin-degrading bacteria. Int J Adv Multidiscip Res 1:84–87
Mishra M, Thakur IS (2010) Isolation and characterization of alkalotolerant bacteria and optimization of process parameters for decolorization and detoxification of pulp and paper mill effluent by Taguchi approach. Biodegradation 21:967–978
Oliveira PLd, Duarte MCT, Ponezi AN, Durrant LR (2009) Use of bacillus pumilus CBMAI 0008 and Paenibacillus sp. CBMAI 868 for colour removal from paper mill effluent. Braz J Microbiol 40:354–357
Peter J, Vandana P, Masih H, Kumar Y (2014) Production optimization and partial purification of laccases from bacterial consortium. Int J Eng Res Technol (IJERT) 3:0181–2278
Piabh K (1990) Determination of lignin in sulphide pulping liquor. Pap Trade J III:35–36
Ravikumar K, Pakshirajan K, Swaminathan T, Balu K (2005) Optimization of batch process parameters using response surface methodology for dye removal by a novel adsorbent. Chem Eng J 105:131–138
Renugadevi R, Ayyappadas M, Preethy P, Savetha S (2011) Isolation, screening and induction of mutation in strain for extra cellular lignin peroxidase producing bacteria from soil and its partial purification. J Res Biol 4:312–318
Rinaldi R, Jastrzebski R, Clough MT, Ralph J, Kennema M, Bruijnincx PC, Weckhuysen BM (2016) Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew Chem Int Ed 55:8164–8215
Sasidharan S, Achuthan T, Tamilselvan E, Raju S, Rengaraju B (2015) Synthesis of copolymer from lactic acid-polyethylene terephthalate (LA-PET) copolymerization. Afr J Biotech 14:953–960
Singh S, Chandra R, Patel D, Reddy M, Rai V (2008) Investigation of the biotransformation of pentachlorophenol and pulp paper mill effluent decolorisation by the bacterial strains in a mixed culture. Biores Technol 99:5703–5709
Vares T, Kalsi M, Hatakka A (1995) Lignin Peroxidases, Manganese peroxidases, and other ligninolytic enzymes produced by phlebia radiata during solid-state fermentation of wheat straw. Appl Environ Microbiol 61:3515–3520
Verma P, Madamwar D (2003) Decolourization of synthetic dyes by a newly isolated strain of Serratia marcescens. World J Microbiol Biotechnol 19:615–618
Xu Z, Qin L, Cai M, Hua W, Jin M (2018) Biodegradation of kraft lignin by newly isolated Klebsiella pneumoniae, Pseudomonas putida, and Ochrobactrum tritici strains. Environ Sci Pollut Res 25:1–11
Yadav S, Chandra R (2015) Syntrophic co-culture of Bacillus subtilis and Klebsiella pneumonia for degradation of kraft lignin discharged from rayon grade pulp industry. J Environ Sci 33:229–238. https://doi.org/10.1016/j.jes.2015.01.018
Acknowledgements
The author (NG) heartily acknowledges Mr. Santanu Sasidharan (Department of Biotechnology, National Institute of Technology Warangal, Telangana, India) for helping in submission of 16S rRNA sequence to NCBI. We also thank Batul Diwan (Department of Biotechnology, National Institute of Technology Raipur, Chhattisgarh, India) for teaching response surface methodology (RSM). Lastly, we thank Aayush Kukreja (Department of Biotechnology, RGPV Bhopal, M.P, India) for helping me with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gaur, N., Narasimhulu, K. & Pydi Setty, Y. Extraction of ligninolytic enzymes from novel Klebsiella pneumoniae strains and its application in wastewater treatment. Appl Water Sci 8, 111 (2018). https://doi.org/10.1007/s13201-018-0758-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0758-y