Abstract
This study aimed to investigate the biological activities of Lactobacillus gasseri SM 05 (L. gasseri) and Lacticaseibacillus casei subsp. casei PTCC 1608 (L. casei) in the black raspberry (Rubus dolichocarpus) juice (BRJ) environment, and also the anti-adhesion activity against Salmonella typhimurium (S. typhimurium) in fermented black raspberry juice (FBRJ). Results showed significant anti-adhesion activity in Caco-2 epithelial cells. In the anti-adhesion process, lactic acid bacteria (LAB) improve intestinal health by preventing the adhesion of pathogens. Adding LAB to BRJ produces metabolites with bacteriocin properties. Major findings of this research include improved intestinal health, improved antidiabetic properties, inhibition of degradation of amino acids, and increase in the nutritional value of foods that have been subjected to heat processing by preventing Maillard inhibition, and inhibition of oxidation of foodstuff by increased antioxidant activity of BRJ. Both species of Lactobacillus effectively controlled the growth of S. typhimurium during BRJ fermentation. Moreover, in all tests, as well as Maillard's and α-amylase inhibition, L. gasseri was more effective than L. casei. The phenolic and flavonoid compounds increased significantly after fermentation by both LAB (p < 0.05). Adding Stevia extract to FBRJ and performing the HHP process showed convenient protection of phenolic compounds compared to heat processing.
Similar content being viewed by others
Introduction
Berries are edible and juicy fruits that have significant biological properties such as antioxidant and anti-inflammatory characteristics. The berries’ compounds are also very useful for the skin. It is a remarkable feature of these fruits that contain vitamin C, and this vitamin greatly prevents cataracts, arthritis, and macular degeneration1. Among the fruits of berry, Raspberries, in terms of vitamin C, are very rich and prevent colds and boost the immune system2,3. In some previous studies due to the presence of carotenoids4 and α-linolenic acid, tocopherols, linoleic acid and finally oleic acid in raspberry, this fruit is known as having health promotion properties5,6. The compounds that protect cells against oxidation are phenolic and flavonoid compounds (resveratrol and apijanine and routine, etc.) as well as anthocyanins, ellagitannins, lignans and tannins in this fruit7. Phenols in raspberry also prevent disorders such as Alzheimer, obesity, Parkinson and diabetes8,9. Dark or black berries have more antioxidants than red berries and these high antioxidants have a direct relationship with the phenolic and flavonoid compounds of these fruits10.
LAB were seen and described in milk for the first time in 1780. They utilize fermentative carbohydrates to form lactic acid11,12. The benefits of lactic fermentation include controlling some cancers, preventing the development of intestinal infections, improving the nutritional value of some foods, and preventing the increase in cholesterol levels in the body. Furthermore, as fermentation in dairy products leads to the reduction of lactose sugar, and it could also be useful for some people who have an intolerance to lactose13.
Among the LABs, Lactobacillus gasseri and Lacticaseibacillus casei have therapeutic indications; for example, a study reported L. gasseri improved stress and anxiety in healthy people as well as sick people with intestinal problems14. Moreover, L. gasseri BNR17 was effective in weight loss15 and L. gasseri G098 reduced colitis caused by dextran sodium sulfate16 and prevented the growth of bacteria and viruses in the vaginal environment17. L. casei variety rhamnosus has been used to treat or prevent diarrhea18. It is also reported that if foods containing L. casei-01 are consumed continuously, it prevents increasing blood pressure and blood sugar19.
Pathogens are invaders that attack the cells of the host body. Pathogens have a wide range of characteristics, some of them attack a single host and others can attack a wide range of hosts20. Salmonella enterica serovar typhimurium is a primary intestinal pathogen that infects animals as well as humans. The infection begins by eating contaminated food or water so that salmonellae colonize the intestinal epithelium and cause gastrointestinal diseases. Salmonella has especial clinical importance in both developed and developing countries and is the cause of most common diarrheal diseases transmitted through food. Several outbreaks of Salmonella serovars are reported annually as S. enterica serovar typhimurium and S. enterica serovar enteritidis as the most common etiological agents21.
There are important reasons that the combination of these two bacteria with BRJ has been used:
-
1.
According to the nutritional grade of the two bacteria used, L. gasseri has been isolated from the fermentation of camel milk. L. casei has also been isolated from Kraft Swiss cheese. Also, by improving the antioxidant properties of BRJ, the harmful effects of oxidation in the body and food can be prevented.
-
2.
High medicinal properties of these two bacteria: these two important LABs potentially have functional and valuable properties that have high therapeutic and medicinal potential and so far, it has not been investigated in BRJ, and its combination with BRJ can improve health by producing useful metabolites at the end of fermentation and produce an important functional drink in terms of health promotion because it can be useful and effective against harmful bacteria, such as salmonella, which are involved in intestinal diseases.
-
3.
Due to the compatibility of these bacteria with the food environment and good growth in BRJ and the high fermentation power of simple sugars and nutrients in fruit juice, combining each of the LABs with BRJ produces a new product and this inoculation by increasing phenolic and flavonoid content in BRJ improves the functional and nutritional properties of BRJ. Maintaining these properties over time using Stevia extract is another goal of this research, and the Stevia plant has the following characteristics.
Stevia rebaudiana Bertoni is one of the two species that produces glycosides in addition to its sweetening properties22. This plant has also many biological and therapeutic properties, including anti-oxidant, anti-diabetic, anti-obesity, anti-tumor, etc.23. Due to the reduction of sugar in the fermentation process by two LABs and also, considering the high sweetness properties of the Stevia plant and its therapeutic properties, the extract of this plant was used along with different HHP and VAT pasteurization methods.
Therefore, this study was performed to investigate the antagonistic and anti-adhesion effects of the mentioned Lactobacillus strains on S. typhimurium during the fermentation of BRJ. The biological activities of FBRJ, including inhibition of the Maillard reaction, and antioxidant and antibacterial properties were also investigated. The stability of the phenolic and flavonoid compounds and the antioxidant strength of the samples were examined during 28 days of storage in the presence of Stevia extract.
Results and discussion
Viable cells and pH
Figure 1a,b show the growth trend of bacteria and pH changes in FBRJ. Initially, the bacteria were inoculated with 5.5 log CFU/mL in BRJ. On the second day of fermentation, the fermentation process was performed slowly and steadily. But, on the first day of fermentation, bacterial production was also much higher than the second day of fermentation. For L. gasseri its value reached 10 log CFU/mL and for L. casei it was 8.8 log CFU/mL at the end of 48 h of fermentation. The pH was also four at the beginning of fermentation and during the fermentation of BRJ containing both LABs, pH decreased to 2.7 for L. gasseri and 2.9 for L. casei. In 2021, Li et al. reported that different strains of L. fermentum were inoculated with 7.5 log CFU/mL in blueberry juice24. By the end of 48 h of fermentation, the number of LAB in all subspecies had increased by more than 35% and some bacteria had reached more than 10 log CFU/mL24. In this study, a more than 81% increase in bacterial population was observed for L. gasseri and a 60% increase was observed for L. casei.
(a) Changes in cell counts of Lactobacillus strains in FBRJ; (b) changes in pH of BRJ during fermentation for 48 h; (c) changes in cell counts of S. typhimurium in various BRJ samples; (d) anti-adhesion assay (inhibition and competition of S. typhimurium in the presence of L. gasseri and L. casei strains) (different capital letters show the significant difference (p ≤ 0.05).
Two strains of Lactobacillus were used in BRJ against S. typhimurium. As shown in Fig. 1b, L. gasseri was effective in reducing pH compared to other treatments that pH reached 3.3 after 24 h and at the end of the fermentation process was 2.7, while pH reduction in fruit juice containing S. typhimurium did not occur during the experiment. It is noteworthy that due to the activity of each lactic bacterium alone in BRJ, the pH rate was lower than when each lactic bacterium was used in combination with S. typhimurium. Of course, this indicates acid production by LAB.
As shown in Fig. 1c, the changes in S. typhimurium count along with any of the tested Lactobacillus are different compared to BRJ and S. typhimurium without LAB. During the fermentation period where the LAB and S. typhimurium are present, the number of pathogenic bacteria increases in the first 24 h of fermentation. But after 24 h, the number of S. typhimurium decreases until the end of the fermentation process. When only pathogenic bacteria without LAB were present in BRJ, until the end of 48 h of fermentation, the number of S. typhimurium continuously increased. The mechanisms of the antibacterial activity of Lactobacillus strains appear to be multifactorial25. Lactobacillus strains inhibit the growth of pathogenic bacteria and sometimes even kill them by lowering the pH by producing acetic and lactic acid26.
Anti-adhesion effects of L. gasseri and L. casei against S. typhimurium
Intestinal infections initiate with the adhesion of the pathogenic bacteria to the mucosal surface. This process occurs through distinguishing the specific receptors by bacteria. Probiotic strains can adhere to gastrointestinal tract (GIT) receptors and block them from pathogens to prevent adhesion27,28. Competition between probiotics and pathogens for the same receptors can be a reason for balancing the GIT microbiota and preventing the infection. In this study, in the competition method after the simultaneous addition of L. gasseri and S. typhimurium, the adhesion rate of S. typhimurium decreased. The same process happened to L. casei to a lesser extent. Antimicrobial compounds such as hydrogen peroxide, bacteriocins, organic acids, and polysaccharides can reduce the adhesion. Other reasons include competition for nutrition and receptors27.
As shown in Fig. 1d, inhibitory effects on S. typhimurium were 44.5 and 32.9% for L. gasseri and L. casei, respectively. Competition effects on S. typhimurium were 54.1 and 41.6% for L. gasseri and L. casei, respectively. Currently, many studies show that L. casei may improve inflammatory bowel disease (IBD) by regulating the balance of intestinal microbiota and the host’s immune response and several researchers also reported that L. casei remarkably impedes the adhesion and invasion of pathogenic bacteria in the gut of food animals29. In addition to L. casei’s anti-adhesion and anti-inflammatory properties, it is compatible with food environment and has shown remarkable anti-diabetic properties, for example, L. casei NRRL-B-1922 has the high ability to grow and adapt in a Punica granatum juice environment, and showed acceptable antidiabetic properties30. Adhesion can be associated with different forms, which include the adhesion of these bacteria together or the adhesion of bacteria to host cells. An effective method is to prevent the formation of biofilm and colonization and thus prevent the initiation of infection by bacteria. Probiotic bacteria can compete nutritionally with other pathogens through the good adhesion ability to host's intestinal cells, and by bindings to the receptor, they take the chance of adhesion from the pathogens. Moreover, antimicrobial compounds such as hydrogen peroxide, which is an oxygen catabolite, and bacteriocins, which are composed of antimicrobial protein substances, as well as organic acids such as lactic acid and acetic acid, are all involved in the anti-adhesion potential31.
In one of the studies that related to the anti-adhesion properties of Lacticaseibacillus rhamnosus GG, KU200656 and Lactiplantibacillus plantarum KU200656, Staphylococcus aureus (S. aureus) ATCC 6538 were able to work on pathogens such as Staphylococcus aureus, Listeria monocytogenes, Escherichia coli (E. coli) and Salmonella typhimurium to have 25–78% anti-adhesion properties31.
Another study showed that the consumption of L. casei WLCA02 significantly reduced the amounts of Salmonella in the ileum, colon and cecum and also in the liver and spleen that are outside the intestine, and this reduction was also observed in the feces32.
ABTS assay
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) is a free radical used to measure antioxidant power. Phenolic compounds in fruit or vegetable juices give hydrogen to a strong oxidizing agent such as potassium persulfate in this reaction33. As indicated in Fig. 2a, at the beginning of fermentation, the rate of absorption of free radicals was 31% for both LABs. With the progress of fermentation, the absorption of ABTS radical for L. gasseri and L. casei strains reached 58 and 56.5% on the first day and 74 and 72.5% on the second day of fermentation of BRJ, respectively. There was a significant difference between different fermentation hours (0, 6, 12, 24, 36, and 48) in terms of antioxidant power to remove free radicals at the 5% level, but in each time of fermentation, there was no significant difference between L. gasseri and L. casei at the α = 5% level.
(a) ABTS scavenging for L. gasseri and L. casei; (b) antioxidant properties (FRAP) of BRJ fermented by Lactobacillus strains; (c) total phenolic content (mg/L) for L. gasseri and L. casei; (d) total flavonoid content (mg/L) of BRJ for L. gasseri and L. casei; (e) α-amylase inhibition of BRJ by L. gasseri and L. casei; (f) effect of Lactobacillus strains on Maillard reaction inhibition (%) during the fermentation of BRJ (different capital letter show the significant difference (p ≤ 0.05)).
In 2019, the improvement of the antioxidant power of apple juice by L. plantarum (ATCC 14917) was investigated and the increase of antioxidant power was reported by DPPH and ABTS methods at fermentation times of 24, 48 and 72 h. The reason for this increase in antioxidant power was found to be due to the increase in the amount of phenolic and flavonoid compounds in apple juice during fermentation34.
FRAP assay
The FRAP method is a process for measuring the antioxidant power based on the oxidation and reduction system. In this method, iron in the complex (FeIII-TPTZ) receives electrons in an acidic environment and as a result of this reaction, the color of the solution turns from yellow to blue35,36. The concentration of Fe2+ ions formed in the solution is found by measuring the absorbance at the wavelength of 593 nm. Higher absorption values in spectrophotometer indicate higher antioxidant power37. According to Fig. 2b, the result of the FRAP assay of BRJ at the end of 48 h of fermentation increased significantly compared to the beginning of fermentation. In this experiment, L. gasseri had higher antioxidant power than L. casei and at the end of 48 h FRAP antioxidant activities reached 9.9 and 9.5 mol/L for L. gasseri and L. casei, respectively. Similarly, in a recent study by Chen et al., L. acidophilus and L. plantarum improved the color and antioxidant activity of Strawberry during fermentation using the FRAP method38. At the end of 48 h of fermentation, it reached 14.54 and 14.52 mol/L for L. acidophilus and L. plantarum, respectively.
The antioxidant properties of LAB can be related to various factors, such as antioxidant enzymes resulting from their activity, exopolysaccharides and bioactive peptides produced by them, and finally manganese ions. In addition, beneficial bacteria in the intestine can produce bioactive food antioxidants using various enzymatic reactions39. In other researches, it is stated that LAB adapted to the environment of beverages such as jujube juice and Djulis can increase the absorption of free radicals, and thus the antioxidant properties improve through the increase of phenolic compounds40,41. In some studies, the effect of organic acids on antioxidant properties has been observed, such as lactic, caproic, acetic, lauric, and capric acid, which had increased significantly after the fermentation of jujube-wolfberry composite Juices and have increased the antioxidant properties42. Furthermore, phlorizin and caffeic acid increased antioxidant properties in apples after fermentation by LAB43. According to these studies, remarkable antioxidant properties were observed for LAB after the fermentation.
Total phenolic and total flavonoid content
In BRJ, the total phenolic and flavonoid content was 605 mg/L and 13 mg CE/L, respectively (Fig. 2c,d) and both LABs were able to significantly increase the total phenolic and total flavonoid content. For example, the amount of phenolic and flavonoid compounds reached from 788 to 958.33 mg/L and 37.5 to 53.5 mg CE/L after 24 and 48 h of fermentation for L. gasseri, respectively. As specified in the Fig. 2c,d, L. gasseri was stronger than L. casei. For L. casei, the amount of phenolic and flavonoid compounds reached 923.66 mg/L and 51.5 mg CE (Catechin)/L at the end of 48 h of fermentation. These results match the previous research. Previous studies have found that fermentation can increase compounds such as kaempferol and quercetin and other phenolic and flavonoid compounds44. Also, the previous studies indicated the effect of fermentation time on increased phenolic compounds in okra seed, which reached the highest level (1460 mg GAE/100 g) after 24 h of fermentation (1460 mg GAE/g), while at the beginning of fermentation, it was at its lowest (185 mg GAE/100 g)45.
There are different opinions about the increase in the amount of phenolic compounds due to the fermentation of LAB, but what is common among the theories is the activity of enzymes secreted by LAB. For example, in one study, Lactobacillus could produce a wider range of phenolic acids through ring fission, deglycosylation, reductive metabolism of phenyl acyl fragments, aromatic dehydroxylation and hydroxylation, decrease of double bonds (carbon–carbon) and all these operations were performed on anthocyanins38. In addition, in the fermentation process by Lactobacillus in Jussara pulp, secreted β-galactosidase and α-galactosidase enzymes were involved in the destruction and conversion of anthocyanins46.
In addition, in another research, it was reported that as a result of the activity of enzymes that are released in the fermentation process, compounds such as tannins, flavonoids, phenylpropanoid, and alkaloids are produced in a greater amount. In the fermentation process, LAB polymerizes complex phenolic compounds and converts them into simple phenolic compounds. It has been mentioned in a study that natural fermentation using microorganisms increases acidity and β-glucosidase produced by L. plantarum via the hydrolysis of complex phenolic compounds that enable them to produce simple phenolic compounds in high amounts47.
HPLC analysis
HPLC analysis of phenolic compounds of BRJ after 48 h of fermentation by L. gasseri is shown in Table 1. Among these 10 compounds, anthocyanins have a significant contribution in terms of quantity. Ellagic acid and cyanidin 3-glucoside had the highest amount among phenolic compounds and chlorogenic acid had the lowest amount. The order of phenolic compounds was as follows:
Ellagic acid > Cyanidin 3-glucoside > Gallic acid > Ferulic acid > Caffeic acid > Quercetin > Kaempfrol > p-Coumaric acid > Myricetin 3-glucoside > Chlorogenic acid.
Finally, the results of this study are consistent with the previous studies, as it has been reported that ellagic acid had the highest amount (2875 mg/kg) among other phenolic compounds of Raspberry leaf extract48.
α-Amylase inhibition
Compounds that inhibit the α-amylase enzyme prevent the increase of blood glucose and even reduce it after eating. In fact, they do this by slowing down the breakdown of carbohydrates in the small intestine and reducing postprandial hyperglycemia49. As seen in Fig. 2e, at the beginning of fermentation, the inhibition rate of the α-amylase enzyme was 15.5%, and with the start of the fermentation process by two LABs, the inhibition rate increased to a greater extent. At the end of 48 h of fermentation, the inhibition rate reached 48 and 46% for L. gasseri and L. casei bacteria, respectively. α-Amylase inhibitors prevent the absorption of starch from foods in the body, so-called a starch blocker. Because starch is a carbohydrate with a complex structure, it is not easily absorbed by the body unless it is broken down by α-amylase or other related enzymes50.
In previous studies, it has been stated that the inhibition of α-amylase by Morindalucida leaf extract may be due to the presence of several compounds present in the plant such as tannins, flavonoids and saponins49. In another study, α-amylase and α-glucosidase inhibitory activity of six groups of flavonoids were investigated and it was found that from the category of flavonols, myristin 64%, Quercetin 50%, Kaempferol 18% and Cyanidins from the category of anthocyanins inhibited α-amylase by more than 50%51. Therefore, due to the presence of the above compounds in the sample of FBRJ, the increase in inhibition of α-amylase can be related to the flavonols and anthocyanins of BRJ.
Maillard reaction
The Maillard reaction occurs between amino acids and reducing sugars, and brown aromatics compounds and UV-absorbing intermediates are the end products of this reaction52,53. LAB converts sugars into organic acids such as acetic, propionic, and lactic acids54 which leads to an increase in the ratio of non-reducing sugars to reducing sugars in the entire fruit juice environment, thus, the needed primary substance to carry out the Maillard reaction is removed, and the reaction is prevented55. Previous studies have also shown that fructose and glucose levels decreased during fermentation due to the activity of LAB and the consumption of simple sugars55,56. In the present work, L. gasseri and L. casei were able to prevent the Maillard reaction by 47 and 43%, respectively during 48 h of fermentation (Fig. 2f), while in the first of the fermentation, this amount was 6% in BRJ. Compared to the initial time of fermentation, the prevention of Maillard reaction was more effective in the first 24 h of the fermentation than in the second 24 h of the fermentation.
Antibacterial activity
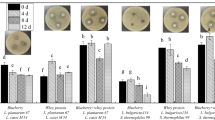
In this study, three samples called fermented black raspberry juice (FBRJ), black raspberry juice (BRJ) and black raspberry Juice + Stevia extract (BRJ + SE) were used. All results were reported in terms of growth inhibition area (mm) in Table 2. All samples had antibacterial properties. E. coli were more sensitive than S. aureus to the samples and a larger inhibition zone was obtained (Table 2). The highest antibacterial power was related to BRJ + SE (25 g/L) against E. coli with diameters of 27 and 28 mm and the lowest was related to BRJ against S. aureus with a diameter of 6.7 and 7 mm, in VAT and HHP processing, respectively. The important point is that due to the fermentation process, the antibacterial activity of all fermented samples by L. gasseri and L. casei increased compared to unfermented samples. These results are consistent with the antibacterial properties of Fuji” apple juice. In that research, it was reported that samples fermented with L. acidophilus, L. casei and L. plantarum had higher antibacterial power than unfermented samples57.
Pasteurization processes and Stevia extract on the stability of phenolic and flavonoid compounds
Stevia extract was added at the rate of 12.5 and 25 g/L to the BRJ samples and pasteurization processes such as HHP and VAT were applied to the samples. The amount of phenolic and flavonoid compounds was measured on the 28th day and the amount of reduction of these compounds after 28 days was also calculated to determine the exact effect of extract and pasteurization processes on phenolic and flavonoid compounds. As shown in Fig. 3a,b, all fermented and unfermented raspberry samples mixed with Stevia extract at two levels of 12.5 and 25 g/L and subjected to different pasteurization processes have a significant difference from each other on day 28 in terms of the amount of phenolic compounds. The fermented and unfermented sample of BRJ that was mixed with Stevia extract at a level of 25 g/L and subjected to the HHP process (FBRJ + SE 25 (HHP)) and (BRJ + SE 25 (HHP)) was the highest in terms of the amount of phenolic compounds, and the fermented and unfermented sample of BRJ, which was mixed with Stevia extract at the level of 12.5 g/L and was subjected to VAT process (FBRJ + SE 12.5 (VAT)) and (BRJ + SE 12.5 (VAT)) were the lowest in terms of phenolic compounds. The order of the values of phenolic compounds is shown in Fig. 3a,b.
(a) Total phenolic compounds of FBRJ samples combined with Stevia extract on the 28th day; (b) total phenolic compounds of unfermented BRJ samples combined with Stevia extract on the 28th day; (c) total flavonoid contents of FBRJ samples combined with Stevia extract on the 28th day; (d) total flavonoid contents of unfermented BRJ samples combined with Stevia extract on the 28th day; (e) simultaneous effect of concentration and pasteurization process on phenolic compounds (FBRJ) after 28 days; (f) simultaneous effect of concentration and pasteurization process on phenolic compounds (unfermented BRJ) after 28 days (different capital letters show the significant difference (p ≤ 0.05)).
Regarding the remaining flavonoid compounds after 28 days, the results were according to phenolic compounds with slight differences. There was a significant difference between all samples, except FBRJ + SE 12.5 (Control) and FBRJ + SE 25(VAT) samples, as well as between BRJ + SE 25 (VAT) and BRJ + SE 12.5 (Control) samples that there was no significant difference between the mentioned samples. There was also no significant difference in terms of quantity and the last-mentioned samples were even completely equal in terms of quantity (Fig. 3c,d).
The order of strength of samples for flavonoid contents is as follows:
The simultaneous effect of concentration and pasteurization process on phenolic compounds after 28 days can be seen in Fig. 3e,f, and the HHP process is the best process for pasteurization of fermented and unfermented samples of BRJ with Stevia extracts at different levels. The VAT pasteurization sample was so destructive that even the amount of phenolic compounds remained in this method was lower than the control sample, and these amount at the end of 28 days for the fermented samples that were combined with 25 and 12.5 g/L of Stevia were 820 and 700 mg/L, respectively and in the HHP process 1180, 1080 and 1110 and 1012 mg/L were reported for the control sample, which indicates the positive effect of the HHP process and the negative effect of the VAT process on the pasteurization of phenolic compounds. The same trend prevailed for non-fermented samples; the only difference was that the non-fermented samples that were combined with Stevia extract were at a lower level than the non-fermented samples in terms of the amount of remaining phenolic compounds. For BRJ + SE 25 (HHP) and BRJ + SE 12.5 (HHP) and BRJ + SE 25 (Control) and BRJ + SE 12.5 (Control) and BRJ + SE 25 (VAT) and BRJ + SE 12.5 (VAT), respectively, the values of 735, 675, 637, 592, 570, 487 mg/L were obtained and as it is known, the higher concentration of Stevia extract is more reliable in the stability of phenolic compounds than lower concentrations. The same effect was seen for flavonoid compounds. These results are consistent with the results of Rios-Corripio et al. They investigated the HHP process with different pressures and times for the stability of phenolic and flavonoid compounds and anthocyanins of fermented pomegranate juice. They compared the HHP process with VAT (63 °C/30 min) and HTST (72 °C/15 s) methods, as well as the control. HHP method, HTST, control and VAT were the best to worst pasteurization processes to preserve phenolic and flavonoid compounds and anthocyanins of fermented pomegranate juice, respectively58.
As shown in Fig. 4a–d, the amount of reduction of phenolic and flavonoid compounds for fermented and non-fermented samples of BRJ without adding Stevia extract is higher than in other samples. For example, for the sample fermented by L. gasseri and the non-fermented sample to which no extract was added, the reduction of phenolic compounds was observed by 223 and 260 mg/L, respectively, which was the largest reduction among the samples. For the fermented and non-fermented samples of BRJ to which 25 g/L of Stevia extract was added and subjected to the HHP process, 70 and 100 mg/L reduction of phenolic compounds was observed, respectively, which was the lowest decrease among the samples. Also, in the case of flavonoids, a decrease in flavonoid compounds was observed by 9.5 and 10.5 mg CE/L, respectively, for the sample fermented by L. gasseri and non-fermented to which no extract was added, which had the largest decrease among the samples. For the fermented and non-fermented samples of BRJ to which 25 g/L of Stevia extract was added and subjected to HHP process, 5.5 and 6.5 mg/L reduction of flavonoid compounds was observed, which was the lowest reduction among the samples. It can be concluded that increasing the concentration of Stevia extract along with the HHP process can help the stability of phenolic and flavonoid compounds after 28 days of storage in the refrigerator. In a study, the stability of phenolic, antioxidant, and antidiabetic compounds of roselle drink was investigated using Stevia and citric acid; It was reported that the addition of Stevia to the beverage increased the stability of gallic acid, rosmarinic acid, epigallocatechin gallate, and quercetin during storage for 12 days. It was also reported about anthocyanins that the content of total anthocyanin after storage had a slight increase in the content of pelargonidin-3-glucoside, cyanidin-3-glucoside, and peonidin-3-glucoside after storage and these compounds are pigments that have an important effect in increasing the antioxidant property in fruit juice and over time due to the changes that occur in the B-ring of anthocyanins compounds, 3,4-dihydroxybenzoic and phydroxybenzoic, 3,4,5-trihydroxybenzoic acids are produced and one of the characteristics of the produced compounds is that they are hydrogen donors and inhibit the activity of free radicals and thus increase the antioxidant activity. In addition, they increase the amount of total phenolic compounds during storage59. According to the above, for this research, such a result can be taken in relation to the changes in anthocyanins and the increase of phenolic acids in BRJ.
(a) Decrease phenolic compound for FBRJ samples during 28 days; (b) decrease phenolic compound for unfermented BRJ samples during 28 days; (c) decrease flavonoid content for FBRJ samples during 28 days; (d) decrease flavonoid content for BRJ samples during 28 days (different capital letters show the significant difference (p ≤ 0.05)).
In addition, in previous research conducted on Stevia, it has been determined that this plant has many glycosides and is mainly composed of steviol, which can link with the surrounding sugars or glycosides with these glycosidic bonds60. Therefore, it can have a protective role on the phenolic compounds of BRJ over time. Another study showed that glycosides and pigments in Stevia have been preserved to a significant extent even after bread processing and have preserved the antioxidant property of bread61; therefore, Stevia has many pigments that can bond with the pigments of BRJ and create a protective complex.
It has been reported that phenolic compounds extracted from plants have medicinal properties, including anti-diabetic properties and anti-inflammatory properties62,63. It has also been reported that the extraction of phenolic compounds in three forms, free, esterified and insoluble-bound, from (Cucumaria frondosa) tentacles significantly control the amount of secondary oxidation products in fish64. On the other hand, raspberries have valuable terpenes such as α-pinene, β-myrcene and linalool, and among these compounds65, it has been reported that α-pinene, in addition to having therapeutic properties such as protecting the heart and being anti-cancer, also has flavoring properties, so the extract and essential oil of this fruit together with Stevia can be used as a natural preservative in food for a long time66.
Conclusion
In conclusion, fermentation by LAB is an effective method to increase the biological properties of BRJ with low concentration and both L. gasseri and L. casei bacteria can have a significant effect on the growth inhibition of S. typhimurium and its adhesion to intestinal cells. In addition, the results show that the amount of total phenolic compounds such as gallic acid, cinnamic acid, and ferulic acid increases due to the addition of LAB to BRJ. Subsequently, it was found in the antioxidant tests that the antioxidant power of BRJ has also increased significantly. Also, the increase in the FRAP power and ABTS scavenging assay and α-amylase inhibition caused by these two LABs in BRJ made it a special product, and L. gasseri was more effective than L. casei in increasing the biological properties of fruit juice. Considering the reduction of natural sugars in BRJ, due to fermentation and the sweetening properties, and high antioxidant and antibacterial power of Stevia extracts, adding Stevia extract to FBRJ and performing the HHP process to protect as much phenolic compounds as possible on the resulting mixture can create an excellent product in terms of phenolic and flavonoid compounds which can maintain these biological properties to an acceptable level for up to 28 days. Also, considering the survival of these two bacteria after 48 h of fermentation in fruit juice and also considering the dairy origin of these two bacteria, they can be a suitable option for use in food products, especially dairy products. Protection of increased phenolic and flavonoid compounds due to the fermentation of L. gasseri and L. casei bacteria in BRJ during 28 days of storage at refrigerator temperature is a new method that has not been carried out so far. The combination of Stevia plant extract and the use of the appropriate HHP method in fermented and non-fermented BRJ, and obtaining positive results regarding the protection of the phenolic compounds of BRJ in a long period of preservation are the significant findings of this study that have not been reported so far.
Experimental
Materials and instruments
Lactobacillus gasseri SM 05, Lacticaseibacillus casei subsp. casei (PTCC 1608) and Salmonella enterica subsp. enterica serotype typhimurium (PTCC 1709) were purchased from the Institute of Industrial Fungi and Bacteria of Iran. Caco-2 cells and Lactobacillus strain were prepared according to Behbahani et al.27. The pH was measured with a pH meter (WTW Bench pH meter 7110, Germany). High performance ternary gradient liquid chromatography system was used for the identification of major phenolic compounds (Azura model from Knauer Co. made in Germany), equipped with P6.1L pump and MWD 2.1 UV detector.
Preparation and fermentation of black raspberry juice
Plant sample collection was in accordance with relevant institutional, national, and international guidelines and legislation. Collection of raspberry samples was according to IUCN policy statement on research involving species at risk of extinction. As it was not a conserved species, permission for collection and also growth location of the plant was obtained from the agricultural office of Guilan province and the minimum necessary amount was harvested. After the collection of raspberry samples, waste materials were separated from each fruit. It is important to note that the used species from BRJ in this work was R. dolichocarpus. The fruit was washed, homogenized, and filtered through sterilized gauze to obtain the juices. BRJ was prepared according to Li et al.24 with slight modifications. The pH of the juice was adjusted to about 4 with 1 M Na2CO3. The amount of Brix in the juice was adjusted to 15, and then the juice was pasteurized at 80 °C for 5 min. In the next stage, the inoculation of juice was started with different bacteria (1% v/v). The initial microbial count was approximately 5.5 log colony forming units (CFU)/mL. Juice samples inoculated with bacteria were kept in the dark at 37 °C for 48 h and following biological tests were performed at the intervals of 0, 6, 12, 24, 36, and 48 h. To evaluate the activity of LAB and pathogens in the fruit juice, the following was done: L. gasseri, L. casei and S. typhimurium were added to juice at about 5.5 log CFU/mL. The control sample was BRJ containing Salmonella without LAB that incubated at 37 °C for 48 h and then the bacteria were counted.
Extraction of Stevia extract
Stevia rebaudiana Bertoni was obtained from the farms of Guilan province and Stevia extract was obtained using the extraction method67 by pure water solvent. After extraction, the extract was concentrated and used fresh to protect the phenolic compounds of BRJ within 28 days. Some experiments including measurement of total phenolic and total flavonoid compounds, and antibacterial activity were performed on Stevia extract in combination with BRJ.
Determination of viable cells
Viable cell counts were obtained by serial dilution with sterile peptone water until 10–6 dilution. Aliquots of 0.1 mL of dilution were plated, in triplicate in plates containing MRS Agar (spread plate method). The plates were incubated for 48 h at 30 °C. Plates containing 30–300 colonies were measured and recorded as colony forming units (CFU) per 1 mL of solution68.
Anti-adhesion activity of L. gasseri and L. casei against S. typhimurium
This experiment was done with two approaches: competition and inhibition. In the competition approach, strains of L. gasseri and L. casei and S. typhimurium were added to Caco-2 cells simultaneously (in equal proportions) and incubated for 1 h and the unbound bacteria were washed with sterilized PBS. Then, this work was done once without LAB and the percentage of competition for adhesion was calculated according to the formula of Behbahani et al.27.
In the inhibition approach, the first two LABs were incubated for 1 h after being added to Caco-2 cells and the unbound bacteria were washed with sterilized PBS immediately Salmonella was added to the container and after 1 h of incubation, the washing step was carried out. After that, the cells and bacteria were separated with Triton (X-100, 0.05%), and the percentage of inhibition of its adhesion was calculated according to the formula of Behbahani et al.27.
ABTS radical scavenging assay
ABTS assay of BRJ was prepared according to the literature34. Briefly, 2.45 mM potassium persulfate was mixed with 7 mM ABTS solution, and the mixture was kept at 25 °C for 12 h before use. The dilution of ABTS·+ solution was prepared with ethanol and obtained an absorbance of 0.70 at 734 nm. Next, 0.3 mL from samples of BRJ reacted with diluted ABTS·+ solution (5 mL) and absorbance were read after six min at 734 nm. Then, RSA was calculated according to the following formula:
where A0 is the absorbance of ABTS radical solution without sample and As is the absorbance of the sample34.
FRAP assay
Briefly, at first FRAP reagent (1.8 mL) was blended with distilled water (0.2 mL), then the solution was mixed with 50 µL BRJ and incubated for 10 min at 37 °C and immediately the absorbance of the resulting mixture was read at 593 nm by spectrophotometer based on mol of Fe2+/L69.
Total phenolic content
Specifically, 0.2 mL samples (BRJ during fermentation) or (BRJ + Stevia) with proper dilution were mixed with 1.5 mL of tenfold-diluted Folin-Ciocalteu reagent and 1.5 mL of 7.5% (w/v) sodium carbonate. After holding at room temperature in darkness for 40 min, the absorbance was read at 765 nm using a UV–Vis spectrophotometer. The total phenolic content was standardized against Gallic acid and expressed as mg Gallic acid equivalents/L24.
Total flavonoid content
Determination of total flavonoid content was performed according to Salarbashi et al. method. Total flavonoid content was expressed as mg catechin equivalents/L70.
High performance liquid chromatography (HPLC) analysis
For this purpose, 2 mL of BRJ was transferred to a 10 mL flask and made up to 10 mL with methanol. The mixture was homogenized for 20 min at room temperature in an ultrasonic bath. Then, it was centrifuged for 15 min at a speed of 5000 rpm. The clear methanolic solution was passed through a 0.45-µm filter and then used for analysis with the HPLC device according to the below conditions. The chromatographic system comprised a C18 column (Zorbax Extend-C18, 5 μm, 250 × 4.6 mm, Agilent). Mobile phase: acetonitrile, Buffer: 0.5% v/v acetic acid, injection volume: 20 µL and pump program: start from 10% acetonitrile, rising to 100% during 35 min. The flow rate was 1 mL/min and diode-array detection was performed at 280 nm71.
α-Amylase inhibition
The α-amylase solution was obtained according to the literature72. Briefly, 1 mL of BRJ samples were mixed with starch solution (3.5 mL, 1%), α-amylase solution (4 mL), 3,5-dinitrosalicylic acid and distilled water (10 mL) and incubated for 15 min at 30 °C. The reaction stopped by sodium carbonate (200 µL, 0.1 M). The absorbance was read at 540 nm. The α-amylase inhibitory activity was calculated using the formula:
Maillard inhibition
Inhibition of Maillard reaction was measured based on Hashemi et al.55.
Antibacterial activity
First, for fermented samples, the filtration of fermented juice was performed by a nominal 0.22 mm filter. Then, 20 µL of the BRJ or Stevia extract (25 g/L) combined with BRJ was poured on sterile disks (6 mm diameter) and placed on the Muller-Hinton agar containing 107 bacterial cells per mL under aseptic conditions. Afterward, the plates were incubated (37 °C and 24 h). After incubation, the diameter around the disks (zones of inhibition) was measured73,74.
Effect of pasteurization processes and Stevia extract on the stability of phenolic and flavonoid compounds
Different processes were used to measure the stability of phenolic compounds during 28 days. At first, the HHP process was used according to Cantúa et al. method, and based on the results obtained in relation to phenolic compounds in this study, a pressure of 600 MPa was used for 10 min at room temperature75. Also, the pasteurization method called VAT (63 °C/30 min) was used58. In addition to using these processes, the extract of Stevia rebaudiana Bertoni plant at two levels of 12.5 and 25 g/L was also used to evaluate the stability of phenolic compounds in different samples of BRJ (fermented and unfermented).
Statistical analysis
The data were presented as the mean ± standard deviation (SD). All the experiments were performed in triplicate. The one-way analysis of variance (ANOVA) was used to compare the means by SAS software (version 9.4). To evaluate whether the fermented BRJ samples were different from the control, in most assays, their difference percentage was calculated and significant differences between the two fermented juices were obtained using the student t-test. A significant level was considered as p value ≤ 0.05.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Suvetha, K. & Shankar, M. Types and importance of berries—a review. Am. J. Biol. Pharm. Res. 1(2), 46–48 (2016).
Ponder, A. & Hallmann, E. The nutritional value and vitamin C content of different raspberry cultivars from organic and conventional production. J. Food Compos. Anal. 87, 103429 (2020).
Kumar, D. & Rizvi, S. I. Significance of vitamin C in human health and disease. Ann. Phytomed. 1, 9–13 (2012).
Li, X. et al. Characterization of carotenoids and phenolics during fruit ripening of Chinese raspberry (Rubus chingii Hu). RSC Adv. 11, 10804–10813 (2021).
Hendawy, O. et al. Cold-pressed raspberry seeds oil ameliorates high-fat diet triggered non-alcoholic fatty liver disease. Saudi Pharm. J. 29, 1303–1313 (2021).
Marić, B. et al. Recovery of high-content ω–3 fatty acid oil from raspberry (Rubus idaeus L.) seeds: Chemical composition and functional quality. LWT-Food Sci. Technol. 130, 109627 (2020).
Oluwole, O. et al. Role of phenolic acid, tannins, stilbenes, lignans and flavonoids in human health—a review. Int. J. Food Sci. Technol. https://doi.org/10.1111/ijfs.15936 (2022).
Kallscheuer, N. et al. Identification and microbial production of the raspberry phenol salidroside that is active against Huntington’s disease. Plant Physiol. 179, 969–985 (2019).
Tsuda, T. Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants 5, 13 (2016).
Kostecka-Gugala, A. et al. Antioxidant properties of fruits of raspberry and blackberry grown in central Europe. Open Chem. 13, 1313–1325 (2015).
Sharma, R. et al. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 6, 106 (2020).
Castillo Martinez, F. A. et al. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 30(1), 70–83. https://doi.org/10.1016/j.tifs.2012.11.007 (2013).
Das, T. K. et al. Current status of probiotic and related health benefits. Appl. Food Res. 2(2), 100185. https://doi.org/10.1016/j.afres.2022.100185 (2022).
Nishida, K. et al. Health benefits of Lactobacillus gasseri CP2305 tablets in young adults exposed to chronic stress: A randomized, double-blind, placebo-controlled study. Nutrients 11, 8 (2019).
Mahboubi, M. Lactobacillus gasseri as a functional food and its role in obesity. Int. J. Med. Rev. 6, 59–64 (2019).
Zhang, W. Q. et al. The Lactobacillus gasseri G098 strain mitigates symptoms of DSS-induced inflammatory bowel disease in mice. Nutrients 14(18), 3745 (2022).
Mei, Z. & Li, D. The role of probiotics in vaginal health. Front. Cell. Infect. Microbiol. 12, 963868 (2022).
Lai, H.-H. et al. Probiotic Lactobacillus casei: Effective for managing childhood diarrhea by altering gut microbiota and attenuating fecal inflammatory markers. Nutrients 11(5), 1150 (2019).
Colombo Pimentel, T. et al. Health benefits and technological effects of Lacticaseibacillus casei-01: An overview of the scientific literature. Trends Food Sci. Technol. 114, 722–737 (2021).
Ribet, D. & Cossart, P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 17(3), 173–183. https://doi.org/10.1016/j.micinf.2015.01.004 (2015).
Mkangara, M. Prevention and control of human Salmonella enterica infections: An implication in food safety. Int. J. Food Sci. 2023, 8899596. https://doi.org/10.1155/2023/8899596 (2023).
Lemus-Mondaca, R. et al. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 132, 1121–1132 (2012).
Khilar, S. et al. An Insight into attributes of Stevia rebaudiana Bertoni: Recent advances in extraction techniques, phytochemistry, food applications and health benefits. J. Agric. Res. 10, 25 (2022).
Li, S. et al. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: Fermentation characteristics and evolution of phenolic profiles. Chemosphere 276, 130090 (2021).
Chen, C.-C. et al. Antimicrobial activity of Lactobacillus species against carbapenem-resistant enterobacteriaceae. Front. Microbiol. https://doi.org/10.3389/fmicb.2019.00789 (2019).
Van Zyl, W., Deane, S. & Dicks, L. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes 12, 25. https://doi.org/10.1080/19490976.2020.1831339 (2020).
Alizadeh Behbahani, B., Noshad, M. & Falah, F. Inhibition of Escherichia coli adhesion to human intestinal Caco-2 cells by probiotic candidate Lactobacillus plantarum strain L15. Microb. Pathog. 136, 103677 (2019).
Oerlemans, M. M. P. et al. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J. Funct. Foods 76, 104289. https://doi.org/10.1016/j.jff.2020.104289 (2021).
Deng, Z. et al. Lactobacillus casei protects intestinal mucosa from damage in chicks caused by Salmonella pullorum via regulating immunity and the Wnt signaling pathway and maintaining the abundance of gut microbiota. Poult. Sci. 100(8), 101283. https://doi.org/10.1016/j.psj.2021.101283 (2021).
Mustafa, S. M. et al. Kinetic profile and anti-diabetic potential of fermented Punica granatum juice using Lactobacillus casei. Process Biochem. 92, 224–231. https://doi.org/10.1016/j.procbio.2020.01.014 (2020).
Lee, J.-E., Lee, N.-K. & Paik, H.-D. Antimicrobial and anti-biofilm effects of probiotic Lactobacillus plantarum KU200656 isolated from kimchi. Food Sci. Biotechnol. https://doi.org/10.1007/s10068-020-00837 (2020).
Tian, L. et al. Preventive of Lacticaseibacillus casei WLCA02 against Salmonella Typhimurium infection via strengthening the intestinal barrier and activating the macrophages. J. Funct. Foods 104, 105507. https://doi.org/10.1016/j.jff.2023.105507 (2023).
Rumpf, J., Burger, R. & Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 233, 123470. https://doi.org/10.1016/j.ijbiomac.2023.123470 (2023).
Li, Z. et al. Enhanced antioxidant activity for apple juice fermented with Lactobacillus plantarum ATCC14917. Molecules 24(1), 51 (2018).
Zhong, Y., & Shahidi, F. Methods for the assessment of antioxidant activity in foods. In Handbook of Antioxidants for Food Preservation. 2015, 287–333.
Soural, I. et al. Different values obtained by the FRAP method for the determination of slowly and rapidly reacting phenols. Acta Aliment. 51(1), 84–92 (2022).
Balaji, K. et al. Determination of total phenolic, flavonoid content and antioxidant activity of terminalia chebula (Fruit). Res. J. Pharm. Biol. Chem. Sci. 6, 413–417 (2015).
Chen, W. et al. Improvement in color expression and antioxidant activity of strawberry juice fermented with lactic acid bacteria: A phenolic-based research. Food Chem. 17, 100535 (2022).
Łepecka, A. et al. Antioxidant activity of environmental lactic acid bacteria strains isolated from organic raw fermented meat products. LWT Food Sci. Tech. 174, 114440. https://doi.org/10.1016/j.lwt.2023.114440 (2023).
Li, T. et al. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 339, 127859. https://doi.org/10.1016/j.foodchem.2020.127859 (2021).
Kuo, H. C. et al. Enhanced antioxidant activity of Chenopodium formosanum Koidz. by lactic acid bacteria: Optimization of fermentation conditions. PLoS One https://doi.org/10.1371/journal.pone.0249250 (2021).
Zhao, X. et al. Effect of fermentation by lactic acid bacteria on the phenolic composition, antioxidant activity, and flavor substances of jujube–wolfberry composite juice. Sci. Technol. Aliment. 184, 25 (2023).
Wu, C. et al. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT Food Sci. Tech. 122, 109064. https://doi.org/10.1016/j.lwt.2020.109064 (2020).
Zhao, Y.-S. et al. Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants (Basel) 10(12), 2004 (2021).
Adetuyi, F. O. & Ibrahim, T. A. Effect of fermentation time on the phenolic, flavonoid and vitamin C contents and antioxidant activities of Okra (Abelmoschus esculentus) seeds. Niger. Food J. 32, 128–137 (2014).
Braga, A. R. C. et al. Lactobacillus fermentation of jussara pulp leads to the enzymatic conversion of anthocyanins increasing antioxidant activity. J. Food Compos. Anal. 69, 162–170. https://doi.org/10.1016/j.jfca.2017.12.030 (2018).
Wijayanti, E., N. Candra Eka Setiawan, & Christi, J. Effect of Lactic Acid Fermentation on Total Phenolic Content and Antioxidant Activity of Fig Fruit Juice (Ficus carica). 2017.
Pavlović, A. V. et al. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 87, 304–314 (2016).
Kazeem, M. I., Adamson, J. O. & Ogunwande, I. A. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. Biomed. Res. Int. 2013, 527570 (2013).
Oboh, G., Akinyemi, A. J. & Ademiluyi, A. O. Inhibition of α-amylase and α-glucosidase activities by ethanolic extract of Telfairia occidentalis (fluted pumpkin) leaf. Asian Pac. J. Trop. Biomed. 2(9), 733–738. https://doi.org/10.1016/s2221-1691(12)60219-6 (2012).
Tadera, K. et al. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. (Tokyo) 52(2), 149–153. https://doi.org/10.3177/jnsv.52.149 (2006).
Kuda, T. & Yano, T. Mineral composition of seawater bittern nigari products and their effects on changing of browning and antioxidant activity in the glucose/lysine Maillard reaction. Appl. Biochem. Biotechnol. 172(6), 2989–2997 (2014).
Rakhmanova, A., Khan, Z. & Shah, K. A mini review fermentation and preservation: Role of lactic acid bacteria. MOJ Food Process. Technol. 6, 414–417 (2018).
Lund, M. N. & Ray, C. A. Control of maillard reactions in foods: Strategies and chemical mechanisms. J. Agric. Food Chem. 65(23), 4537–4552 (2017).
Hashemi, S., Jafarpour, D. & Jouki, M. Improving bioactive properties of peach juice using Lactobacillus strains fermentation: Antagonistic and anti-adhesion effects, anti-inflammatory and antioxidant properties, and Maillard reaction inhibition. Food Chem. 365, 130501. https://doi.org/10.1016/j.foodchem.2021.130501 (2021) (Epub 2021 Jul 1).
Wang, Y. et al. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 9, 612285 (2021).
Yang, J. et al. Fermentation and storage characteristics of “Fuji” apple juice using Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus plantarum: Microbial growth, metabolism of bioactives and in vitro bioactivities. Front. Nutr. 9, 833906 (2022).
Rios-Corripio, G. et al. Influence of high hydrostatic pressure processing on physicochemical characteristics of a fermented pomegranate (Punica granatum L.) beverage. Innov. Food Sci. Emerg. Technol. 59, 102249 (2020).
Pérez Ramírez, I. et al. Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage. Food Chem. 172, 885–892. https://doi.org/10.1016/j.foodchem.2014.09.12 (2015).
Simlat, M. et al. The content of phenolic compounds in Stevia rebaudiana (Bertoni) plants derived from melatonin and NaCl treated seeds. Plants 12, 780. https://doi.org/10.3390/plants12040780 (2023).
Ruiz-Ruiz, J. C. et al. Antidiabetic and antioxidant activity of Stevia rebaudiana extracts (Var Morita) and their incorporation into a potential functional bread. J. Food Sci. Technol. 52(12), 7894–7903. https://doi.org/10.1007/s13197-015-1883-3 (2015).
Deka, H., Choudhury, A. & Dey, B. K. An Overview on plant derived phenolic compounds and their role in treatment and management of diabetes. J. Pharmacopuncture 25(3), 199–208. https://doi.org/10.3831/KPI.2022.25.3.199 (2022).
Rahman, M. M. et al. Role of phenolic compounds in human disease: current knowledge and future prospects. Molecules 27(1), 233. https://doi.org/10.3390/molecules27010233 (2022).
Hossain, A. et al. Phenolic profiles of Atlantic sea cucumber (Cucumaria frondosa) tentacles and their biological properties. Food Res. Int. (Ottawa, Ont) 163, 112262. https://doi.org/10.1016/j.foodres.2022.11226 (2023).
Du, X. & Qian, M. Flavor chemistry of small fruits: Blackberry, raspberry, and blueberry. In Flavor and Health Benefits of Small Fruits 27–43 (American Chemical Society, 2010). https://doi.org/10.1021/bk-2010-1035.ch003.
Karimkhani, M. M. et al. Extraction and purification of α-pinene; a comprehensive review. Crit. Rev. Food Sci. Nutr. https://doi.org/10.1080/10408398.2022.2140331 (2022).
Karimkhani, M. M. et al. Effect of extraction solvents on total phenolic compound, lipid peroxidation, antioxidant and cytotoxic activity of leaves of Rubus armeniacus (Himalayan blackberry). J. Food Meas. Charact. 15(6), 5725–5734 (2021).
Yang, F., Wang, Y. & Zhao, H. Quality enhancement of fermented vegetable juice by probiotic through fermented yam juice using Saccharomyces cerevisiae. Food Sci. Technol. 40, 25 (2020).
Hmid, I. et al. Comparative study of phenolic compounds and their antioxidant attributes of eighteen pomegranate (Punica granatum L.) cultivars grown in Morocco. Arab. J. Chem. 20, 113 (2013).
Salarbashi, D. et al. Investigation of optimal extraction, antioxidant, and antimicrobial activities of Achillea biebersteinii and A. wilhelmsii. Pharm. Biol. 50, 1168–1176. https://doi.org/10.3109/13880209.2012.662235 (2012).
Karimkhani, M. M. et al. Antioxidant and antibacterial activity of safflower (Carthamus tinctorius L.) extract from four different cultivars. Qual. Assur. Saf. Crops Foods 8, 1–10. https://doi.org/10.3920/QAS2015.0676) (2016).
Ghauri, S. et al. Assessment of α-amylase and α-glucosidase inhibitory potential of Citrus reticulata peel extracts in hyperglycemic/hypoglycemic rats. 3 Biotech 11(4), 167 (2021).
Karimkhani, M. M. et al. Effect of extraction solvents on lipid peroxidation, antioxidant, antibacterial and antifungal activities of Berberis orthobotrys Bienerat ex CK Schneider. J. Food Meas. Character. 13(1), 357–367. https://doi.org/10.1007/s11694-018-9951-9 (2019).
Akia, M. et al. Antibacterial activity of polymeric nanofiber membranes impregnated with texas sour orange juice. Eur. Polym. J. 115(1), 1–5 (2019).
Buitimea-Cantúa, G. V. et al. Effect of high hydrostatic pressure and pulsed electric fields processes on microbial safety and quality of black/red raspberry juice. Foods 11(15), 2342. https://doi.org/10.3390/foods11152342 (2022).
Acknowledgements
This paper was extracted from a PhD thesis with code: 3/57391 supported by the Research Deputy of Ferdowsi University of Mashhad. The supports from the University of Qom are appreciated.
Author information
Authors and Affiliations
Contributions
M.M.K.: methodology, formal analysis, investigation, writing: original draft, A.J. and M.N.: supervision, investigation, visualization, validation, project administration, writing—review and editing, resources, formal Analysis, M.A.: resources, conceptualization, investigation, S.M.J.: resources, conceptualization, fund acquisition, analysis, T.Z.: conceptualization, investigation, writing: original draft, formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karimkhani, M.M., Jamshidi, A., Nasrollahzadeh, M. et al. Fermentation of Rubus dolichocarpus juice using Lactobacillus gasseri and Lacticaseibacillus casei and protecting phenolic compounds by Stevia extract during cold storage. Sci Rep 14, 5711 (2024). https://doi.org/10.1038/s41598-024-56235-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56235-7
- Springer Nature Limited