Abstract
Autologous matrix induced chondrogenesis (AMIC) is a bone marrow stimulating technique used for the surgical management of chondral defects of the talus. The present study evaluated the clinical outcomes and imaging of AMIC as revision procedure for failed AMIC surgery for osteochondral defects of the talus. Forty-eight patients with symptomatic osteochondral defects who received a revision AMIC were evaluated after a minimum of two years follow-up. Patients with previous procedures rather than AMIC, those who required additional surgical procedures (e.g. ligament repair or deformity correction), or those who had evidence of kissing, bilateral, or multiple lesions were excluded. Outcome parameters included the Visual Analogic Scale (VAS), Tegner Activity Scale, the American Orthopedic Foot and Ankle Score (AOFAS), and the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score. All patients were followed by an assessor who was not involved in the clinical management. 27 patients were enrolled in the present study. The mean age of the patient was 34.9 ± 3.1 years, and the mean BMI 27.2 ± 5.1 kg/m2. The mean defect surface area was 2.8 ± 1.9 cm2. The mean follow-up was 44.3 ± 21.4 months. The mean hospital length of stay was 4.4 ± 1.4 days. At final follow-up, the mean VAS score was 4.1 ± 3.1, the mean Tegner 3.5 ± 1.6, the mean AOFAS 58.8 ± 20.6. The preoperative MOCART score was 22.1 ± 13.7 points, the postoperative MOCART score was 42.3 ± 27.9 points (+ 20.2%; P = 0.04), respectively. 30% (8 of 27 patients) experienced persistent pain and underwent a further chondral procedure. Concluding, AMIC could be a viable option as revision procedure for failed AMIC in recurrent symptomatic osteochondral defects of the talus. The PROMs indicated that patients were moderately satisfied with the procedure, and the MOCART score demonstrated a significant improvement from baseline to the last follow-up. A deeper understanding in prognostic factors and patient selection is critical to prevent failures.
Similar content being viewed by others
Introduction
Focal chondral and osteochondral defects of the talus are common in sports medicine1,2,3. The etiology and long-term natural course of these lesions has not been fully elucidated; some studies suggest only moderate or even no progression of joint degeneration following non-operative management4,5, while others report a concerning progression to frank ankle osteoarthritis even in young patients6. Frequently, symptomatic osteochondral defects of the talus limit sports and daily activities, and may require surgical management7. Lesions refractory to non-surgical care for three to six months may be suitable for surgical management8.
For primary osteochondral talar lesions, a variety of surgical treatments has been proposed9,10,11,12,13,14,15, but the superiority of any surgical technique has not been confirmed16. For lesions smaller than 1.5 cm2, bone marrow stimulation (BMS) techniques provide satisfactory long-term outcomes17, and a pooled success rate of 61% was reported for non-primary lesions18. For larger lesions, both matrix-induced autologous chondrocyte implantation (mACI) and autologous matrix-induced chondrogenesis (AMIC) have been proposed19. Theoretically, mACI should induce a superior repair with type II cartilage, and could be ideal for both primary and revision settings. However, although mACI provides reliable outcomes, these seem to be equivalent to other surgical techniques, but at higher costs20. Osteochondral transplantation techniques have also been suggested as a revision modality for osteochondral lesions of the talus21,22. A major drawback of the technique is the associated donor site morbidity, especially if multiple plugs are harvested23. In recent studies, autograft plugs outperformed allograft tissues in terms of efficacy, chondral wear and the number of secondary procedures24. However, quantitative T2 mapping suggests that even autograft tissue may not mirror native hyaline cartilage25, indicating a potential cellular dedifferentiation of transplanted cartilage over time. Further problems are associated with graft integration and less than ideal restoration of anatomic joint congruence25. For these reasons, AMIC combined with bone grafting has gained further interest, as it might also present a feasible single-stage procedure for revisions, without sacrificing osteochondral tissue from the knee joint. Especially for larger non-primary lesions, published data remain sparse.
Whether repeat AMIC performed in revision settings is feasible has not been studied to date. The present study evaluated the clinical outcomes and imaging of AMIC as revision procedure for failed AMIC surgery for osteochondral defects of the talus. We hypothesised that AMIC could be a viable option as a revision procedure for failed AMIC surgery for osteochondral defects of the talus.
Methods
Patient recruitment
The present study was conducted according to the principles of the Declaration of Helsinki and was approved by the ethics committee of the RWTH Aachen University (project ID EK 305/13). The present study follows the Strengthening the Reporting of Observational Studies in Epidemiology: the STROBE Statement26. In May 2021 the databases of the RWTH University Clinic of Aachen (Germany) and University Hospital of Salerno (Italy) were accessed. All the patients who had undergone AMIC following failed previous AMIC for chondral defects of the talus were retrieved. The inclusion criteria were: (1) failed previous AMIC procedure for focal osteochondral defect, (2) pain lasting > 6 months, (3) failed conservative therapies, (4) pain suggestive of focal chondral defect, (5) have undergone preoperative MRI with suggestive findings, (6) minimum two years follow-up, and (7) signed informed consent. The exclusion criteria were: (1) other previous or concomitant procedures rather than isolated AMIC, (2) kissing lesions, (3) bilateral lesions, (4) multiple lesions, (5) any other relevant pathology that can influence the study, and (6) patients unable to understand the nature of the treatment of the study (e.g. cognitive impairment, language barrier).
Surgical technique
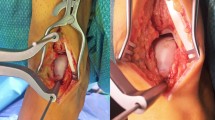
All the surgeries were performed by three experienced surgeons following the same surgical protocol. The ankle was plantar flexed, and a 2 mm Kirscher-wire was drilled in the distal tibia and another one in the talus. A Hintermann spreader (Integra LifeSciences, Plainsboro, NJ) was used for joint distraction. A diagnostic arthroscopy was performed using standard anterolateral and anteromedial portals. The osteochondral defect was identified, and the necrotic surrounding cartilage was shaved until a viable chondral shoulder was reached. Subsequently, a malleolar osteotomy was performed. The subchondral bone was milled until active bleeding was achieved and the defect filled using autologous cancellous bone grafting harvested from the ipsilateral osteotomy site. Subsequently, the residual chondral defect was measured with a metal template. A collagen I/III porcine resorbable membrane (Chondroguide®, Geistlich Pharma AG, Switzerland) was accurately trimmed to slightly oversize the defect. Fibrin glue was used to secure the membrane into the defect. Two malleolar screws were used to fix the malleolar osteotomy. The surgical wound was sutured in a standard fashion. The ankle was flexed and extended several times and the correct positioning of the membrane was confirmed arthroscopically. The postoperative care and rehabilitation protocols followed were conducted according to previously published reports27,28.
Outcomes of interest
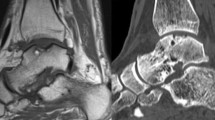
On admission, the following data were recorded: age, gender, side, date of surgeries, additional autologous cancellous bone grafting during the index procedure, BMI (Kg/m2), symptoms duration prior of the revision surgery and length of the hospitalisation. The area of the defect was measured using MRI sequences29. In May 2021, with informed consent, patients were invited to complete the following patient reported outcome measures (PROMs): Visual Analogic Scale (VAS), Tegner Activity Scale, and the American Orthopaedic Foot and Ankle Score (AOFAS). Data on complications and additional procedures were also retrieved. A MRI was performed to every patient at follow-up. The MRI results were evaluated using the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score30. The MOCART score was assessed by a trained radiologist with experience in musculoskeletal imaging and successively double checked by an experienced orthopaedic surgeon. Disagreements between the two assessors were debated and solved by discussion. Patients who did not agree to participate in the clinical assessment and/or imaging examination were excluded from the present investigation. All the patients were followed by an assessor who was not involved in the clinical management of the patients and blinded to MRI results.
Statistical analysis
All statistical analyses were performed by the main author (F.M.) using the software IBM SPSS (Version 25). Continuous variables were analysed with mean difference (MD) and standard error (SE). The confidence interval (CI) was set at 95%. Values of t-test < 0.05 were considered statistically significant.
Ethical approval
The present study was conducted according to the principles of Helsinki and was approved by the ethic committee of the RWTH Aachen University (project ID EK 305/13).
Consent to participate
All patients firmed written consent and willingness to participate to the present study.
Results
Patient recruitment
A total of 48 patients underwent repeat AMIC following failure of previous AMIC of the talus. A total of 14 patients were excluded as they did not match the eligibility criteria: kissing lesions (N = 2), bilateral lesions (N = 2), multiple lesions (N = 6), chronic disease which may influence the outcome (N = 1), follow-up shorter than two years (N = 4). A further 7 patients were excluded because they did not wish to participate in the study. Eventually, 27 patients were enrolled in the present study (Fig. 1).
Patient demographic
63% (17 of 27 patients) were female. The mean time span between the index and the revision surgery was 18.3 ± 5.7 months. The mean follow-up was 44.3 ± 21.4 months. The mean area of the defect was 2.8 ± 1.9 cm2. 63% (17 of 27) of defects were medial, and 10 of 27 were lateral. During the index procedure, 44% (12/27) of patients received an additional autologous cancellous bone graft from the osteotomy site. Patient demographic data are shown in greater detail in Table 1.
Outcomes of interest
At last follow-up, the mean VAS score was 4.1 ± 3.1, the mean Tegner 3.5 ± 1.6, the mean AOFAS 58.8 ± 20.6 (Table 2).
The MOCART score reached 42.3 ± 27.9 points (MD + 20.2%; SE 5.379; 95% CI 9.41–30.98; P = 0.04, Table 3).
One patient experienced a wound infection, which was successfully treated with antibiotics. 30% (8 of 27 patients) experienced persistent pain, and underwent a further chondral procedure. All the patients underwent screws removal at a minimum of 12 months postoperatively. No complications related to the osteotomy were reported.
Discussion
According to the main findings of the present study, repeat AMIC combined with cancellous bone graft may be a viable treatment option for larger (mean 3.2 cm2) osteochondral lesions of the talus in patients in whom a primary AMIC procedure failed. Current evidence on revision procedures following failed surgical management for osteochondral defects is limited, and unpredictable results are reported. The clinical outcomes and imaging of AMIC as revision procedure for a failed AMIC for osteochondral defects of the talus have been scantly investigated. To the best of our knowledge, this is the first patient series detailing the results of repeat AMIC for chondral and osteochondral lesions of the talus.
The single most important indication for reoperation following a repeat AMIC was implant removals after medial malleolar osteotomy. This finding agrees with a recent systematic review, reporting that tissue irritation requiring hardware removal was the most frequent adverse event of the index procedure31. In the current literature, the re-intervention rate of the open AMIC was reported to be 9 to 14%, and age, BMI, and the preoperative performance status of the patients seem to have a detrimental impact on clinical outcomes after surgical treatment of osteochondral lesions of the talus11,28,31,32,33,34. The impact of biological (e.g. age), lesion-specific (location, size, acuity) and biomechanical (e.g. BMI, instability) variables have been reported as prognostic factors35. Yoon et al. suggested that 11% of 399 patients after primary arthroscopic treatment required revision surgery. These data suggest that there is a significant number of secondary and tertiary procedures after failed surgical treatment of osteochondral lesions around the ankle. Unfortunately, the level of evidence of the published reports remains low. AMIC combined with autologous bone grafting can often be performed through an anterolateral or anteromedial arthrotomy, avoiding a malleolar osteotomy36. Furthermore, an all arthroscopic AMIC has been described for talar osteochondral defects37. These recent advances may help to further reduce the number of re-interventions. Lambers et al. evaluated 21 studies (n = 299 patients) with 301 talar OCDs that failed primary surgery38. The treatment strategies included conservative treatment, BMS, retrograde drilling, osteochondral transplantation, cartilage implantation and chondrogenesis-inducing therapies (CIT). Two studies based on AMIC were included, and the calculated success rates were 67% (CI 30–90.3%) and 57% (CI 31.6–78.6%), respectively. In our cohort, 70% of patients required no further procedure addressing the osteochondral defect. In a study evaluating AMIC as both primary and revision procedures, Kubosch et al. reported arthroscopic debridement, retrograde drilling and ligament reconstruction as primary procedures. Valderrabano et al. often combined AMIC with a corrective calcaneal osteotomy, when hindfoot malalignment (≥ 10°) was identified; furthermore, a modified Brostrom-Gould procedure was added in unstable ankles (17/26). Thus, concomitant pathologies and accessory procedures pose a significant impact on patient reported outcomes, and must be considered as confounding variables. We therefore included only patients who received a revision AMIC and had no concomitant procedures other than autologous cancellous bone graft harvested from the proximal tibia or iliac crest. In a recent study by the same group, AMIC demonstrated greater outcomes measures by AOFAS, VAS and Tegner scores at follow-up compared to isolated microfractures9. AMIC has been enhanced using bone marrow aspirate as an adjunct to type I/III type collagen matrix39. Murphy et al. evaluated the matrix-associated stem cell transplantation (MAST) technique applied in patients with larger lesions of the talar dome (15mm2 or greater) and patients with a previous failed attempt at microfracture (n = 21), AMIC (n = 1) or OATS (n = 1). The proposed advantage of this treatment method includes the delivery of mesenchymal stem cells right into the defect and a low donor site morbidity, when compared to ACI or OATS. However, the study did not include revision cases only, patients presented with relatively small defects (mean 1.7cm2), and lacks an MRI follow-up and a control group.
Up to date, for large lesions after failed treatment, no optimal surgical management has been established. For tertiary osteochondral defects, the use of contoured metal implants as salvage procedure has been suggested40. Maiorano et al. reported a series of 12 patients (mean age 39) after metal resurfacing (HemiCAP®; Arthrosurface Inc., Franklin, MA), and observed that pain was still present at follow-up41. They therefore concluded that metal resurfacing might not be considered as valid alternative for treatment of osteochondral defects after failed previous surgery. Ettinger et al. retrospectively evaluated a cohort of 10 patients (mean age 47 years, mean BMI 30), who received a HemiCAP® implantation of the medial talar dome after failed previous surgery42. The authors reported an increased risk for postoperative dissatisfaction and persistent ankle pain in overweight patients. Moreover, ten revisions were performed in 7 patients (70%)42. In contrast, a Danish group reported about 31 consecutive patients (mean age 42.8 years) after HemiCAP® implantation, and described one infection, but good mid-term results and no revisions, these latter defined as conversion to ankle fusion, total ankle prosthesis or revision of the implant43. Especially in young and active individuals, various issues including persisting pain and long-term concerns (implant survival) remain associated with (partial) joint replacement, suggesting that a biological treatment concept should be exploited first. For large primary and secondary osteochondral defects of the medial talus, Kerkhoffs et al. recently suggested the Talar OsteoPeriostic grafting from the Iliac Crest (TOPIC) procedure10. This new biological concept has the advantage to provide of a natural scaffold and to mimic the talus anatomically. The anatomic and press-fit incorporation of the graft may help to treat especially cystic lesions and to avoid peak pressures on the tibial plafond, when compared to metallic implants.
We acknowledge a number of strengths and limitations within our study. First, the cohort included only patients with a previously failed surgical treatment by an open AMIC. Previous studies often included cohorts with heterogeneous surgical first-line treatments or variable additional procedures. As a consequence of these strict inclusion criteria, the number of patients in our study is limited. The rate of patients who were lost at follow-up (7 of 34) may increase the risk of attrition bias and impact negatively our conclusion. However, the follow-up was conducted during the COVID-19 pandemic, and some patients did not wish to participate to the study. The AOFAS score has not been validated for patients with osteochondral defects around the talus. Also, a formal control group is not included and a second look arthroscopy has not been performed. Data on PROMs were not collected pre-operatively. Thus, it was not possible to assess the improvement from baseline to the last follow-up. Furthermore, long-term clinical data concerning the efficacy and safety of AMIC are not yet available. The limitation of missing long-term data generally applies to the majority of treatments, except for primary osteochondral defects treated with arthroscopic BMS produces44. The reported outcomes after repeat AMIC and number of secondary procedures related to hardware removal reflect that the treatment of revision AMIC combined with cancellous bone grafting, if necessary, is a viable option, but may benefit from further developments. These innovative modifications may involve the avoidance of a malleolar osteotomy36, the less invasive penetration of the subchondral bone45, and the augmentation with stem cells (e.g. MAST technique)39, among others. In the light of the discussed literature, each available revision technique has an individual profile of safety, efficacy, invasiveness, donor site morbidity, and durability. Ahead of any revision procedure, a comprehensive analysis of the failure is mandatory to match difficult-to-treat patients and lesions with an appropriate surgical revision procedure. The MOCART score was used; however, the reliability of imaging to assess the clinical outcome after cartilage repair has been criticized46,47,48,49. During the index procedure, 12 patients (44%) received an additional autologous cancellous bone transplantation from the osteotomy site which not healed. The remaining patients received isolated AMIC for chondral defects. Apparently, these patients developed subchondral chondral bone necrosis postoperatively. Whether this may influence the outcome is unclear; however, given the limited sample size included in the present investigation, further considerations or subgroup analyses were not conducted. Most patients underwent conservative management before undertaking revision surgery using AMIC. However, given the heterogeneous nature and the lack of documentation on treatments, further subgroup analyses cannot be conducted. Most patients could not attribute the onset of symptoms to a definite event, declaring to have never fully recovered following the index AMIC. Therefore, the time to failure following the index surgery has not been assessed in the present investigation.
Conclusion
AMIC could be a viable option as revision procedure for failed AMIC in recurrent symptomatic osteochondral defects of the talus. At approximately four years follow-up, the PROMs indicated that patients were moderately satisfied with the procedure, and the MOCART score demonstrated a significant improvement from baseline to the last follow-up. A deeper understanding in prognostic factors and patient selection is critical to prevent failures.
Data availability
The datasets generated during and/or analysed during the current study are available throughout the manuscript.
Abbreviations
- AMIC:
-
Autologous matrix induced chondrogenesis
- MD:
-
Mean difference
- SE:
-
Standard error
- CI:
-
Confidence interval
- VAS:
-
Visual Analogic Scale
- AOFAS:
-
American Orthopedic Foot and Ankle Score
- MOCART:
-
Magnetic resonance observation of cartilage repair tissue
References
Valderrabano, V., Miska, M., Leumann, A. & Wiewiorski, M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am. J. Sports Med. 41(3), 519–527. https://doi.org/10.1177/0363546513476671 (2013).
Weber, C. D. et al. Osteochondral lesions of the talus: Individualized approach based on established and innovative reconstruction techniques. Unfallchirurg https://doi.org/10.1007/s00113-021-00964-1 (2021).
Martijn, H. A., Lambers, K. T. A., Dahmen, J., Stufkens, S. A. S. & Kerkhoffs, G. High incidence of (osteo)chondral lesions in ankle fractures. Knee Surg. Sports Traumatol. Arthrosc. https://doi.org/10.1007/s00167-020-06187-y (2020).
Weigelt, L. et al. Long-term prognosis after successful nonoperative treatment of osteochondral lesions of the talus: an observational 14-year follow-up study. Orthop. J. Sports Med. 8(6), 2325967120924183. https://doi.org/10.1177/2325967120924183 (2020).
Seo, S. S., Kim, C. W. & Jung, D. W. Management of focal chondral lesion in the knee joint. Knee Surg. Relat. Res. 23(4), 185–196. https://doi.org/10.5792/ksrr.2011.23.4.185 (2011).
Ikuta, Y., Nakasa, T., Sumii, J., Nekomoto, A. & Adachi, N. Long-term natural course of the osteochondral lesion of the talus in a child: A case report. J. Foot Ankle Surg. https://doi.org/10.1053/j.jfas.2020.09.008 (2020).
van Dijk, C. N., Reilingh, M. L., Zengerink, M. & van Bergen, C. J. Osteochondral defects in the ankle: Why painful?. Knee Surg. Sports Traumatol. Arthrosc. 18(5), 570–580. https://doi.org/10.1007/s00167-010-1064-x (2010).
Lan, T., McCarthy, H. S., Hulme, C. H., Wright, K. T. & Makwana, N. The management of talar osteochondral lesions: Current concepts. J. Arthrosc. Jt. Surg. 8(3), 231–237. https://doi.org/10.1016/j.jajs.2021.04.002 (2021).
Migliorini, F. et al. Autologous matrix induced chondrogenesis (AMIC) compared to microfractures for chondral defects of the talar shoulder: A five-year follow-up prospective cohort study. Life 11, 3. https://doi.org/10.3390/life11030244 (2021).
Kerkhoffs, G., Altink, J. N., Stufkens, S. A. S. & Dahmen, J. Talar osteoperiostic grafting from the iliac crest (TOPIC) for large medial talar osteochondral defects: Operative technique. Oper. Orthop. Traumatol. 33(2), 160–169. https://doi.org/10.1007/s00064-020-00673-9 (2021).
Weigelt, L., Hartmann, R., Pfirrmann, C., Espinosa, N. & Wirth, S. H. Autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: A clinical and radiological 2- to 8-year follow-up study. Am. J. Sports Med. 47(7), 1679–1686. https://doi.org/10.1177/0363546519841574 (2019).
Silva, A. N., Lim, W. J., Cheok, J. W. G., Gatot, C. & Tan, H. C. A. Autologous collagen-induced chondrogenesis versus microfracture for chondral defects of the knee: Surgical technique and 2-year comparison outcome study. J. Orthop. 22, 294–299. https://doi.org/10.1016/j.jor.2020.06.008 (2020).
Solheim, E., Hegna, J. & Inderhaug, E. Clinical outcome after mosaicplasty of knee articular cartilage defects of patellofemoral joint versus tibiofemoral joint. J. Orthop. 18, 36–40. https://doi.org/10.1016/j.jor.2019.10.010 (2020).
Bennett, C. H. et al. Cartiform Implantation for focal cartilage defects in the knee: A 2-year clinical and magnetic resonance imaging follow-up study. J. Orthop. 24, 135–144. https://doi.org/10.1016/j.jor.2021.02.025 (2021).
Puddu, L. et al. Surgical treatment of talar osteo-chondral lesions with micro-fractures, mesenchymal cells grafting on membrane, or allograft: Mid-term clinical and magnetic resonance assessment. J. Orthop. 21, 416–420. https://doi.org/10.1016/j.jor.2020.08.012 (2020).
Dahmen, J. et al. No superior treatment for primary osteochondral defects of the talus. Knee Surg. Sports Traumatol. Arthrosc. 26(7), 2142–2157. https://doi.org/10.1007/s00167-017-4616-5 (2018).
Rikken, Q. G. H., Dahmen, J., Stufkens, S. A. S. & Kerkhoffs, G. Satisfactory long-term clinical outcomes after bone marrow stimulation of osteochondral lesions of the talus. Knee Surg. Sports Traumatol. Arthrosc. https://doi.org/10.1007/s00167-021-06630-8 (2021).
Dahmen, J. et al. Evidence-based treatment of failed primary osteochondral lesions of the talus: A systematic review on clinical outcomes of bone marrow stimulation. Cartilage https://doi.org/10.1177/1947603521996023 (2021).
Migliorini, F. et al. Matrix-induced autologous chondrocyte implantation versus autologous matrix-induced chondrogenesis for chondral defects of the talus: A systematic review. Br. Med. Bull. 138(1), 144–154. https://doi.org/10.1093/bmb/ldab008 (2021).
Lenz, C. G., Tan, S., Carey, A. L., Ang, K. & Schneider, T. Matrix-induced autologous chondrocyte implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int. 41(9), 1099–1105. https://doi.org/10.1177/1071100720935110 (2020).
Fansa, A. M., Murawski, C. D., Imhauser, C. W., Nguyen, J. T. & Kennedy, J. G. Autologous osteochondral transplantation of the talus partially restores contact mechanics of the ankle joint. Am. J. Sports Med. 39(11), 2457–2465. https://doi.org/10.1177/0363546511419811 (2011).
Azam, A., Forster, M. & Robertson, A. Clinical and radiological outcome for trufit plug in the treatment of chondral and osteochondral lesions at a minimum of 2 years. J. Orthop. 15(1), 47–51. https://doi.org/10.1016/j.jor.2018.01.001 (2018).
Nguyen, A., Ramasamy, A., Walsh, M., McMenemy, L. & Calder, J. D. F. Autologous osteochondral transplantation for large osteochondral lesions of the talus is a viable option in an athletic population. Am. J. Sports Med. 47(14), 3429–3435. https://doi.org/10.1177/0363546519881420 (2019).
Shimozono, Y., Hurley, E. T., Nguyen, J. T., Deyer, T. W. & Kennedy, J. G. Allograft compared with autograft in osteochondral transplantation for the treatment of osteochondral lesions of the talus. J. Bone Jt. Surg. Am. 100(21), 1838–1844. https://doi.org/10.2106/jbjs.17.01508 (2018).
Flynn, S. et al. Autologous osteochondral transplantation for osteochondral lesions of the talus. Foot Ankle Int. 37(4), 363–372. https://doi.org/10.1177/1071100715620423 (2016).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 61(4), 344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008 (2008).
Gotze, C., Nieder, C., Felder, H. & Migliorini, F. AMIC for focal osteochondral defect of the talar shoulder. Life 10, 12. https://doi.org/10.3390/life10120328 (2020).
Gotze, C., Nieder, C., Felder, H., Peterlein, C. D. & Migliorini, F. AMIC for traumatic focal osteochondral defect of the talar shoulder: A 5 years follow-up prospective cohort study. BMC Musculoskelet. Disord. 22(1), 638. https://doi.org/10.1186/s12891-021-04506-z (2021).
Deng, E. et al. Both magnetic resonance imaging and computed tomography are reliable and valid in evaluating cystic osteochondral lesions of the talus. Orthop. J. Sports Med. 8(9), 2325967120946697. https://doi.org/10.1177/2325967120946697 (2020).
Albano, D., Martinelli, N. & Bianchi, A. Evaluation of reproducibility of the MOCART score in patients with osteochondral lesions of the talus repaired using the autologous matrix-induced chondrogenesis technique. Radiol. Med. 122, 909–917 (2017).
Malahias, M. A. et al. Autologous matrix-induced chondrogenesis for the treatment of osteochondral lesions of the talus: A systematic review. Orthop. Rev. 12(4), 8872. https://doi.org/10.4081/or.2020.8872 (2020).
Ackermann, J. et al. Autologous matrix-induced chondrogenesis with lateral ligament stabilization for osteochondral lesions of the talus in patients with ankle instability. Orthop. J. Sports Med. 9(5), 23259671211007440. https://doi.org/10.1177/23259671211007439 (2021).
Ayyaswamy, B. et al. Early to medium term outcomes of osteochondral lesions of the talus treated by autologous matrix induced chondrogenesis (AMIC). Foot Ankle Surg. 27(2), 207–212. https://doi.org/10.1016/j.fas.2020.04.008 (2021).
D’Ambrosi, R. et al. Return to sport after arthroscopic autologous matrix-induced chondrogenesis for patients with osteochondral lesion of the talus. Clin. J. Sport Med. 29(6), 470–475. https://doi.org/10.1097/JSM.0000000000000560 (2019).
Dombrowski, M. E. et al. Conservative management and biological treatment strategies: Proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 39(1 suppl), 9s–15s. https://doi.org/10.1177/1071100718779390 (2018).
Galla, M., Duensing, I., Kahn, T. L. & Barg, A. Open reconstruction with autologous spongiosa grafts and matrix-induced chondrogenesis for osteochondral lesions of the talus can be performed without medial malleolar osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 27(9), 2789–2795. https://doi.org/10.1007/s00167-018-5063-7 (2019).
Baumfeld, T., Baumfeld, D., Prado, M. & Nery, C. All-arthroscopic AMIC(®) (AT-AMIC) for the treatment of talar osteochondral defects: A short follow-up case series. Foot 37, 23–27. https://doi.org/10.1016/j.foot.2018.07.006 (2018).
Lambers, K. T. A. et al. No superior surgical treatment for secondary osteochondral defects of the talus. Knee Surg. Sports Traumatol. Arthrosc. 26(7), 2158–2170. https://doi.org/10.1007/s00167-017-4629-0 (2018).
Murphy, E. P., Fenelon, C., Egan, C. & Kearns, S. R. Matrix-associated stem cell transplantation is successful in treating talar osteochondral lesions. Knee Surg Sports Traumatol Arthrosc 27(9), 2737–2743. https://doi.org/10.1007/s00167-019-05452-z (2019).
van Bergen, C. J. A., Reilingh, M. L. & van Dijk, C. N. Tertiary osteochondral defect of the talus treated by a novel contoured metal implant. Knee Surg. Sports Traumatol. Arthrosc. 19(6), 999–1003. https://doi.org/10.1007/s00167-011-1465-5 (2011).
Maiorano, E. et al. HemiCAP® implantation after failed previous surgery for osteochondral lesions of the talus. Foot Ankle Surg. 27(1), 77–81. https://doi.org/10.1016/j.fas.2020.02.008 (2021).
Ettinger, S. et al. Results of HemiCAP(®) implantation as a salvage procedure for osteochondral lesions of the talus. J. Foot Ankle Surg. 56(4), 788–792. https://doi.org/10.1053/j.jfas.2017.04.001 (2017).
Ebskov, L. B., Hegnet Andersen, K., Bro Rasmussen, P., Johansen, J. K. & Benyahia, M. Mid-term results after treatment of complex talus osteochondral defects with HemiCAP implantation. Foot Ankle Surg. 26(4), 384–390. https://doi.org/10.1016/j.fas.2019.05.003 (2020).
van Bergen, C. J. et al. Arthroscopic treatment of osteochondral defects of the talus: outcomes at eight to twenty years of follow-up. J. Bone Jt. Surg. Am. 95(6), 519–525. https://doi.org/10.2106/JBJS.L.00675 (2013).
Benthien, J. P. & Behrens, P. Nanofractured autologous matrix induced chondrogenesis (NAMIC©)–Further development of collagen membrane aided chondrogenesis combined with subchondral needling: A technical note. Knee 22(5), 411–415. https://doi.org/10.1016/j.knee.2015.06.010 (2015).
ASTM F2706-18. STMfO-CaO-C-TSICiaVM. (ASTM International, 2018). www.astm.org. Accessed Oct 2020.
Blackman, A. J. et al. Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: A systematic review and meta-analysis. Am. J. Sports Med. 41(6), 1426–1434. https://doi.org/10.1177/0363546513485931 (2013).
Shive, M. S. et al. BST-CarGel(R) treatment maintains cartilage repair superiority over microfracture at 5 years in a multicenter randomized controlled trial. Cartilage 6(2), 62–72. https://doi.org/10.1177/1947603514562064 (2015).
Albano, D. et al. Clinical and imaging outcome of osteochondral lesions of the talus treated using autologous matrix-induced chondrogenesis technique with a biomimetic scaffold. BMC Musculoskelet. Disord. 18(1), 306. https://doi.org/10.1186/s12891-017-1679-x (2017).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
F.M.: writing, data analysis, conceptualization, patient assessment; H.S.: patient management; N.M.: supervision; J.E.: supervision; P.L.: patient management; F.H.: supervision, patient management; C.D.W.: writing, patient management. All authors have agreed to the final version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Migliorini, F., Schenker, H., Maffulli, N. et al. Autologous matrix induced chondrogenesis (AMIC) as revision procedure for failed AMIC in recurrent symptomatic osteochondral defects of the talus. Sci Rep 12, 16244 (2022). https://doi.org/10.1038/s41598-022-20641-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20641-6
- Springer Nature Limited
This article is cited by
-
MRI and single-cell RNA sequence results reveal the influence of anterior talofibular ligament injury on osteochondral lesions of the talus
Journal of Orthopaedic Surgery and Research (2024)
-
The efficacy of autologous matrix-induced chondrogenesis (AMIC) for osteochondral lesions of the talus in the mid-long term: a systematic review and meta-analysis
Journal of Orthopaedic Surgery and Research (2024)