Abstract
Purpose
The purpose of this systematic review was to identify the most effective surgical treatment for talar osteochondral defects after failed primary surgery.
Methods
A literature search was conducted to find studies published from January 1996 till July 2016 using PubMed (MEDLINE), EMBASE, CDSR, DARE and CENTRAL. Two authors screened the search results separately and conducted quality assessment independently using the Newcastle–Ottawa scale. Weighted success rates were calculated. Studies eligible for pooling were combined.
Results
Twenty-one studies with a total of 299 patients with 301 talar OCDs that failed primary surgery were investigated. Eight studies were retrospective case series, twelve were prospective case series and there was one randomized controlled trial. Calculated success percentages varied widely and ranged from 17 to 100%. Because of the low level of evidence and the scarce number of patients, no methodologically proper meta-analysis could be performed. A simplified pooling method resulted in a calculated mean success rate of 90% [CI 82–95%] for the osteochondral autograft transfer procedure, 65% [CI 46–81%] for mosaicplasty and 55% [CI 40–70%] for the osteochondral allograft transfer procedure. There was no significant difference between classic autologous chondrocyte implantation (success rate of 59% [CI 39–77%]) and matrix-associated chondrocyte implantation (success rate of 73% [CI 56–85%]).
Conclusions
Multiple surgical treatments are used for talar OCDs after primary surgical failure. More invasive methods are administered in comparison with primary treatment. No methodologically proper meta-analysis could be performed because of the low level of evidence and the limited number of patients. It is therefore inappropriate to draw firm conclusions from the collected results. Besides an expected difference in outcome between the autograft transfer procedure and the more extensive procedures of mosaicplasty and the use of an allograft, neither a clear nor a significant difference between treatment options could be demonstrated. The need for sufficiently powered prospective investigations in a randomized comparative clinical setting remains high. This present systematic review can be used in order to inform patients about expected outcome of the different treatment methods used after failed primary surgery.
Level of evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the treatment of talar osteochondral defects (OCDs), a wide variety of treatment strategies have been reported in the literature. These strategies can be divided into a number of treatment groups: conservative treatment, bone marrow stimulation (BMS), retrograde drilling, osteo(chondral) transplantation, cartilage implantation and chondrogenesis-inducing therapies (CIT). In general, the different surgical treatments of talar osteochondral defects have good results, but although the great majority of defects improve after surgical treatment, a minority of lesions will fail first-line surgical treatment and therefore remain symptomatic [30, 40, 63]. This was exemplified by studies conducted by Yoon et al. [62] and Choi et al. [11], in which 11% of 399 patients and 6.7% out of 120 ankles, respectively, required revision surgery. These numbers indicate that secondary and tertiary surgical treatment for talar OCDs is not uncommon [54].
Although these patients generally represent a therapeutic challenge to the orthopaedic surgeon, most research in the past decades has focused on the treatment of primary talar OCDs. To a lesser extent, researchers in the orthopaedic field have attempted to identify promising surgical treatment options for non-primary lesions [62]. Systematic reviews aspiring to determine the most effective treatment option for talar OCDs so far include patient populations of both primary and non-primary lesions [30, 40, 63]. To our knowledge, no previously published systematic review has exclusively investigated the clinical effectiveness of different surgical treatment options for talar OCDs that have failed primary surgery. Hence, the aim of the present systematic review is to identify the most effective surgical treatment for talar OCDs after failed primary surgery.
Materials and methods
Search strategy
The systematic review was prospectively registered at the PROSPERO register [10].
Electronic databases PubMed (MEDLINE), EMBASE, CDSR, DARE and CENTRAL were screened from January 1996 till July 2016 for potential articles of interest (Appendix 1). Since not all titles or abstracts in these databases clearly describe whether the OCD surgery was primary or secondary, the authors deliberately did not use narrower terms such as “secondary”, “tertiary” or “failed primary” in our search as this would potentially exclude eligible studies. In addition to this, a backward citation chaining strategy was used by scanning the literature lists of suitable studies.
Eligibility criteria and study selection
All studies that assessed the effectiveness of different surgical treatment strategies for previously failed surgical intervention of talar OCDs were included in the systematic review. The criteria for exclusion were primary OCDs, a study cohort of <5 patients, aged <16 (the age around which epiphyseal fusing takes place), concomitant distal tibial lesions and a follow-up of less than 6 months. When necessary, authors were contacted via email to provide separate data for those patients with non-primary lesions only and/or for patients ≥16 years old and/or to exclude concomitant tibial lesions. When no reply was received from an author, contact was attempted once more by a second email. In the cases of further lack of response a third, or when necessary a fourth email was sent. Ultimately, if no response was recorded from the corresponding author, then their specific article was excluded. Independent evaluation of the articles was conducted and then discussed by two reviewers (K.L. and J.D.). In cases of disagreement between the reviewers, the opinion from an independent third investigator (G.K.) was found to be decisive. Studies were not blinded for author, affiliation or source, and no limitations were put on language and publication status of screened articles.
Quality assessment
To assess the quality of the included studies, a Newcastle–Ottawa scale (NOS) modified for talar OCD was used [58]. This scale assesses the methodological quality of non-randomized studies (Appendix 2). Each included study was graded on methodological quality by two independent reviewers (K.L. and J.D.).
Data extraction
The following study and patient characteristics were retrieved: age, gender, number of patients and ankles, symptom duration, location, side, size and stage of the defect, type of surgery, clinical scoring system utilized, history of ankle trauma reported, follow-up duration and the OCD classification staging system reported. Pre-operative and post-operative clinical outcome scores were extracted. Clinical values of the last recorded follow-up were used. The treatment strategy in question was defined to be successful when a good or excellent result was reported at follow-up in combination with an accepted scoring system such as the AOFAS (American Orthopaedic Foot and Ankle Society) Ankle/Hindfoot scale, the FAOS (Foot and Ankle Outcome Score) or the Hannover Scoring System. For clarification, an ankle was considered to be successfully treated when at the last follow-up there was a post-operative AOFAS score of 80 or above [27]. When the FAOS (Foot and Ankle Outcome Score) was used, a score of also 80 or above was regarded to be a successful treatment [50]. When the original ankle score by Mazur et al. [35] was used, a score of 70 or higher was considered to be a successful treatment. In one case, a modified Mazur score was used, which had a 100-point scale. In this case, a score of 80 or higher was considered successful treatment [26].
Statistical analysis
Since a formal meta-regression was not methodologically possible for the included studies (i.e. studies were included with highly different methodological study types and small numbers), it was decided to present the results per study by means of a forest plot. A simple pooling method was used to combine data from different studies that had similar methodologies. 95% binomial proportion confidence intervals were calculated for the success percentages of each study with the Wilson score interval and included in the forest plot (CIA, Confidence Interval Analysis for Windows, version 2.2.0) [8]. To compare between groups with categorical variables, a Fisher’s exact test was used (SPSS version 23.0, IBM Corp.). All reported p values are two-sided, and statistical significance was set at p < 0.05.
Results
Search results
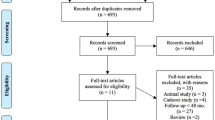
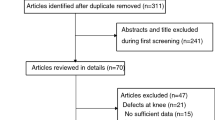
The literature search in the selected databases yielded 1273 articles. After the application of the eligibility criteria to the titles and abstracts, potentially suitable articles were included for full-text review while ineligible articles were deleted. Subsequently, full-text articles were obtained and the eligibility criteria were applied again. After screening and discussion between the first two authors, there was overall consensus in most cases except for two where disagreement persisted, these were resolved via discussion with the senior author. In the end, a total of 21 studies were included (Fig. 1).
Characteristics of included studies (Tables 1, 2, 3)
A total of 299 patients with 301 talar OCDs who failed primary surgery were included. The average age of the patients was 35 (range 18–57), and the percentage of females and males in our study sample was 35 and 65%, respectively. The right ankle was involved in 47% of the cases and the left ankle in 53%. The percentages of medial, lateral, central and combined medial and lateral location involvement were 64, 19, 15 and 2%, respectively. Size of the lesion or surface diameter was rarely described or impossible to extract and was therefore not incorporated into our review. The most frequently used clinical scoring system and osteochondral damage classification system were the American Orthopaedic Foot and Ankle Society Score (AOFAS) and the Berndt and Harty/Loomer Classification system [6, 27]. In total, 17 different scoring systems were found [3, 9, 13, 14, 21, 25, 27, 33, 38, 42, 50, 56, 57, 61] and ten different osteochondral damage classification systems [2, 6, 7, 15, 23, 31, 32, 36, 43, 48] were used (Tables 2, 3). It was only possible to extract Berndt and Harty stages for 80 ankles: there were 0 (0%), 9 (11%), 21 (26%), 26 (33%) and 24 (30%) Berndt and Harty/Loomer [6] stage I, II, III, IV and V cases, respectively. In 87 of the 299 patients, a history of ankle trauma was reported. If divided through the number of treated ankles in the articles that report on history of trauma, this corresponds to 76.3%. The mean follow-up was 40 months [range 12–66].
Methodological quality
Of the 21 studies included, eight were retrospective case series, twelve were prospective case series and there was one randomized controlled trial (RCT). Full consensus was reached between the reviewers regarding the grading of methodological quality. Seventeen studies described that their research was conducted according to protocol. In fourteen studies, the described cohort was truly or somewhat representative to a talar OCD patient sample/population; in the other seven studies the group was either selected by an orthopaedic surgeon or there was no description of the cohort. In all studies, outcome was assessed through independent blind assessment or record linkage. In nine articles, the follow-up was complete or the loss to follow-up was reported to be smaller than 5%. In the other twelve articles, either the percentage loss to follow-up was more than 5% or the follow-up rate was not stated.
Treatment strategies
The treatment strategies were divided into the four previously indicated treatment groups: bone marrow stimulation (debridement and/or drilling), osteo(chondral) transplantation (autograft transfer, allograft transfer and mosaicplasty), cartilage implantation (MACI and ACI) and chondrogenesis-inducing techniques (AMIC). Two studies did not correspond to any of these groups and are presented separately (Fig. 2). All calculated success percentages with their respective confidence intervals are also visually presented in a forest plot (Fig. 3). The calculated success percentages of the pooled groups are shown in an additional forest plot (Fig. 4).
Forest plot of all included studies with the success rates per separate study sorted on treatment strategy and alphabetical order accompanied by number of ankles and follow-up duration (the size of the diamond representing the success rate is categorically adjusted for number of ankles included in the publications)
Forest plot of the success rates after pooling sorted on treatment strategy accompanied by the number of included studies, the success rate with confidence interval and the total number of ankles per group (the size of the diamond representing the success rate is categorically adjusted for the number of ankles included)
Bone marrow stimulation (BMS)
Two studies described the outcome of BMS as a secondary treatment for a total of 25 treated ankles [47, 51]. One of these studies (Savva et al.) solely reported the results of repeat arthroscopic treatment [51]. The authors used the Berndt and Harty classification to grade the OCDs, but excluded cystic lesions as their treatment type (debridement) had been demonstrated to have poor outcomes in these type of lesions [49]. The second study by Reilingh et al. is an RCT investigating the effects of pulsed electromagnetic fields (PEMF) on debridement and microfracture [47]. Due to the fact that the authors indicated that there was no significant difference between the randomized treatment groups, patients from both groups were included. In this study, cystic lesions were not excluded. The two studies describe a post-operative AOFAS of 81 and 80. Success percentages of 75% (debridement alone) and 69% (debridement and microfracture) were calculated with confidence intervals of 47–91% and 42–87%, respectively.
Osteo(chondral) transplantation
Osteochondral transplantation was the most commonly described procedure after failed primary surgery as eight studies were identified describing 135 patients corresponding to 137 treated ankles [1, 5, 12, 16, 20, 22, 45, 60]. Five of these studies were retrospective in design, and the other three were prospectively described case series. The mean follow-up for these studies was calculated to be 4 years (range 17 months–5.5 years). The included studies described different osteo(chondral) transplantation techniques. Three articles investigated a procedure with a classic osteochondral autograft transfer system (OATS) [1, 16, 60]. Two studies described a (autogenous) mosaicplasty procedure, which is similar to an OATS technique but uses multiple grafts creating a mosaic pattern [12, 22]. Three other studies described an allograft procedure [5, 20, 45]. A calculated success rate for all osteochondral transplantation techniques combined is not possible since the study designs were not similar enough for all studies. However, coincidentally all studies describing the allograft procedure were of the same level of evidence (retrospective case series), and all studies describing the autograft procedure and the two describing the mosaicplasty procedure were of the same level of evidence (prospective case series). It was therefore possible to use a simplified pooling method to combine data from these groups (Fig. 4). The autograft group after simple pooling consisted of 73 treated ankles and the allograft group of 38 treated ankles. This gave a calculated mean success rate for the autograft procedure of 90% [CI 82–95%] and a calculated mean success rate of 55% [CI 39.7–69.9%] for the allograft procedure. The success rate of the two studies describing a mosaicplasty procedure which was autogenous in nature for both studies was 65% [CI 46.2–80.6%]. A Fisher’s exact test showed no difference between mosaicplasty and the allograft transfer procedure (p = 0.449). A comparison between these two groups and the aforementioned autograft group was not performed since a mosaicplasty or an allograft procedure is often used for larger OCDs and a direct comparison would not be appropriate.
Cartilage implantation
In total, seven suitable studies were found that described a form of cartilage implantation after failed primary surgery [18, 19, 26, 29, 39, 52, 59]. The two major groups were the classic autologous chondrocyte implantation (ACI) and the more recently developed matrix-associated chondrocyte implantation (MACI). In total, four studies described a MACI procedure [19, 29, 39, 52] and two described an ACI procedure [18, 59]. One study described both an ACI and a MACI procedure [26]. The latter was retrospective in design. All other studies in the cartilage implantation group were prospective, which allowed a simplified pooling method for both the MACI and ACI groups. Eighty-seven ankles were included in the cartilage implantation group as a whole and 39 of those received a MACI procedure and 48 received an ACI procedure. When combining data on the ankles from the studies methodologically suitable for pooling, thirty-three ankles in the MACI group and twenty-two in the ACI group were found. The calculated success rates were 72% [CI 56–85%] and 59% [CI 39–77%] for the MACI and ACI groups, respectively. A Fisher’s exact test showed no significant difference between these two groups.
Chondrogenesis-inducing techniques (CIT)
Two studies that investigated the effects of a chondrogenesis-inducing technique were identified [28, 53]. Both are retrospective in design and performed an autologous-matrix-induced chondrogenesis (AMIC) procedure. The study by Kubosch et al. describes a procedure where the proximal tibia was used as a harvest site [28]. The study by Valderrabano et al. describes the iliac crest as a site to obtain the cancellous bone [53]. In total, 21 patients with 21 treated ankles were described in the two included studies. Both Kubosch and Valderrabano report high mean post-operative AOFAS scores of 91 and 86 points, respectively. The corresponding calculated success rates were 67% [CI 30–90.3%] and 57% [CI 32.6–78.6%], respectively. Both articles also included pre- and post-operative VAS scores for pain, which decreased from 8 and 5.5 to 1.7 and 2, respectively.
Other
Two studies described a specific technique that could not be incorporated into any of the groups. The first article describes a novel metal implant in 20 patients after failed previous treatment [55]. Almost all lesions (18 out of 20) were classified as type V (cystic) lesions [6]. The defect was debrided after which the metal implant was introduced into the defect, and this occurred 2 years (mean) after the last failed surgical treatment. The median AOFAS score improved from 62 points pre-operatively to 87 points post-operatively. Of the nineteen patients, fourteen were successfully treated according to the AOFAS, resulting in a success percentage of 74% [CI 51–88%]. The second study included in this group was a transplantation method using a bone graft [24]. As the bone graft was vascularized, the authors decided not to include this study in the previously described osteochondral transplantation group. For the vascularized bone graft, they identified the medial condyle of the femur as an ideal site to harvest since it is sufficiently large and solid and has a contour similar to the talar surface. Data solely on the non-primary lesions were obtained, which included thirteen patients. The mean VAS for pain decreased from 5.8 to 1.7. The mean AOFAS hindfoot score increased from 66 points pre-operatively to 81 points post-operatively. Nine of the thirteen patients scored good to excellent according to the AOFAS which resulted in a success rate of 69% [CI 42–87%].
Discussion
To the best of our knowledge, this is the first systematic review investigating the effectiveness of treatment options for talar osteochondral defects after failed primary surgical treatment. This is in contrast to previous reviews that focused either solely on primary or both primary and non-primary talar OCDs [30, 40, 63]. The differences in treatment approach for non-primary-treated OCDs often differ and are mainly based on expert opinion since evidence is limited. By means of the present review, it was the goal to give an evidence-based insight into the most effective surgical treatment option.
One of the major differences compared to studies focused solely on primary talar OCDs is that the most described treatment option is different. As for the healing of articular cartilage injuries, O’Driscoll postulated that the treatment options of these defects can be grouped according to four principles. It can be restored, replaced, relieved or resected [41]. It is generally expected that the first-line surgical treatment should at least incorporate restoration of the cartilage, which is normally accomplished by enhancing the intrinsic capacity of this cartilage and subchondral bone to heal itself. In a previous review on primary OCDs, BMS (including retrograde drilling) was the most described treatment method. Not unexpectedly, the most frequently described treatment method for secondary talar OCDs was not BMS but a type of replacement strategy.
In the literature, numerous treatment recommendations are given considering failed primary surgery, for example that patients with failures of previous arthroscopic treatment should be treated with osteochondral transplantation [17]. The recommendations are, however, not based on any concrete data. In this review, the success rates of BMS as a revision surgery were found to be 69 and 75%, which seems acceptable and lies within the success rate ranges found in primary OCD surgery. It, however, lies below the confidence interval after pooling of eleven retrospective studies describing primary surgery in 317 ankles which yielded an overall BMS success rate of 82% [CI 78–86%]. In the present review, only 25 ankles treated with BMS were evaluated. The calculated success rate is, however, still promising, and since BMS is a relatively non-invasive and inexpensive treatment, it should still be considered for certain patients with small secondary defects or at least be included in the shared decision process with the patient when discussing further treatment options after a previously failed primary operation [46]. It should also be noted that a study by Yoon et al. [62]—which was not included in the present review—described a clinically inferior outcome which corresponded to a calculated success rate of 32%. In this study, the authors compared repeat arthroscopy to an osteochondral autograft procedure with the latter obtaining a success rate of 82%; however, in this patient populations were also ankles included with concomitant tibial lesions (5% in the osteochondral transplantation group and 14% in the repeat arthroscopy group). Since an associated tibial lesion was part of the exclusion criteria and despite multiple requests, it was not possible to obtain the separate data, and therefore, this article had to be excluded. Another remark that has to be made in the light of the study by Yoon et al. [62] is that the authors included talar OCDs with subchondral cysts (up to an incidence of 64% in the repeat arthroscopy group), while in the study by Savva et al. [51] subchondral cysts were deliberately excluded. As stated above, an underlying cyst is associated with inferior outcomes, and this could therefore be a plausible reason for the difference observed in the success rates. Thus, when considering performing a BMS procedure after failed previous surgical treatment, the presence of a cyst should be taken into account when choosing the optimal treatment strategy.
Most of the studies described a more invasive osteochondral or cartilage transplantation method as a subsequent procedure for a previously unsuccessfully treated OCD. In the pooled group, the OATS procedure with an autograft showed the highest success rate. Interesting enough, the pooled success rate of this OATS group which combined 73 treated OCDs resulted in a 90% success rate, which was significantly higher than the 77% success rate in our review about primary-treated OCDs (p = 0.0296). The observed difference was a little unexpected and might be explained by differences in size of the treated lesions and concomitant damage to the cartilage of the rest of the ankle joint which were not stated in most studies. Differences in outcome expectancy from the patients can also play a role since the used outcome scores (AOFAS) have a subjective component. Finally, it must be noted that the pooled success rates in the review about primary surgery were all retrospective case series, which were compared to three prospective case series in this review. It is therefore not possible to say whether the difference is due to a clinical or methodological difference.
A difference was found in the autograft versus the allograft procedure with success rates of 90 and 55%, respectively. Differences in these procedures have been previously highlighted in the literature [4, 34, 35, 44]. Since an allograft procedure is typically used for larger defects, it is to be expected that a difference in success percentage was likely to be found. The substantial difference underlines the recommendation that, if possible, an autograft procedure deserves preference over an allograft procedure. The same is the case for the mosaicplasty procedure, in most cases autogenous in nature but also used for larger defects. When comparing the allograft procedure with mosaicplasty, no significant difference was found, and therefore, it is not possible to indicate whether one has superiority in treating larger defects.
One of the major strengths of our systematic review is the contacting with the authors of included studies with the goal of acquiring separate data. This, however, also resulted in a limitation: almost half of the extracted data concerning outcome and success percentages was acquired through the direct approach of the authors which made it virtually impossible to collect all the variables initially being desired to collect in the constructed data set, such as complications reported, lesion size, or classification systems used.
As for pooling of the data, it was not possible to perform a formal meta-regression, that is, utilizing mixed-effects logistic regression in order to compare treatment groups. This is because the number of patients included in the studies was substantially lower than required to obtain stable parameters estimates for this type of analysis [37]. Instead, the authors decided to pool data through a simplified manner where different patients from the same treatment group were added and a new success percentage was calculated. This means that the results presented in the review need to be interpreted with caution. When comparing two different treatment groups, one cannot with certainty state that the difference observed was based on clinical differences or on methodological differences. For example, since the allograft technique is mainly used for the larger defects, it will consequently give a worse outcome.
As for the outcome measurements, the AOFAS score was the most frequently used scoring system. This score as with all the other scores used for success percentage calculation is not officially validated for the clinical evaluation of the treatment of talar OCDs as such. Subsequently, the calculated success percentages have to be interpreted with care. This is clearly exemplified by the outcome reported by the study of Johnson et al. [26]. The adjusted Mazur score was unsatisfactory in the majority of patients, which resulted in a rather low success percentage. However, they found a high average subjective patient-based satisfaction score. This again brings up the question to what extent we should rely on these questionnaires.
The majority of the included studies were of low methodological quality. As long as no randomized comparative clinical trials are conducted (such as mosaicplasty versus allograft transplantation or OATS versus AMIC), data will remain insufficient to draw any firm conclusions. These results should therefore not be used in making decisions about technique but rather for prediction of outcome. In clinical practice, this review can be used to illustrate the different treatment techniques and to give patients an indication about the expected success percentages of the different treatment methods for talar OCDs after failed primary surgery.
Conclusions
In conclusion, multiple diverse surgical treatment options are used for talar OCDs in the case of primary surgical failure. As expected, relatively more invasive methods are administered in comparison with primary treatment. Because of the low level of evidence and the scarce literature reporting on solely non-primary surgery, no methodologically proper meta-analysis could be performed, and it is therefore inappropriate to draw firm conclusions. Besides an expected difference in outcome between the autograft transfer procedure and the more extensive procedures of mosaicplasty and the use of an allograft, neither a clear nor a significant difference between treatment options could be demonstrated. The need for sufficiently powered prospective investigations in a randomized comparative clinical setting remains substantially high.
References
Al-Shaikh RA, Chou LB, Mann JA, Dreeben SM, Prieskorn D (2002) Autologous osteochondral grafting for talar cartilage defects. Foot Ankle Int 23:381–389
Anderson IF, Crichton KJ, Grattan-Smith T, Cooper RA, Brazier D (1989) Osteochondral fractures of the dome of the talus. J Bone Joint Surg Am 71:1143–1152
Barber-Westin SD, Noyes FR, McCloskey JW (1999) Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med 27:402–416
Baums MH, Schultz W, Kostuj T, Klinger HM (2014) Cartilage repair techniques of the talus: an update. World J Orthop 5:171–179
Berlet GC, Hyer CF, Philbin TM, Hartman JF, Wright ML (2011) Does fresh osteochondral allograft transplantation of talar osteochondral defects improve function? Clin Orthop Relat Res 469:2356–2366
Berndt AL, Harty M (1959) Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am 41:988–1020
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85(Suppl II):58–69
Brown LD, Cai TT, DasGupta A (2001) Interval estimation for a binomial proportion. Stast Sci 16:101–133
Carlsson AM (1983) Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 16:87–101
Chien PF, Khan KS, Siassakos D (2012) Registration of systematic reviews: PROSPERO. BJOG 119:903–905
Choi WJ, Park KK, Kim BS, Lee JW (2009) Osteochondral lesion of the talus: Is there a critical defect size for poor outcome? Am J Sports Med 37:1974–1980
de l’Escalopier N, Barbier O, Mainard D, Mayer J, Ollat D, Versier G (2015) Outcomes of talar dome osteochondral defect repair using osteocartilaginous autografts: 37 cases of Mosaicplasty(R). Orthop Traumatol Surg Res 101:97–102
Domsic RT, Saltzman CL (1998) Ankle osteoarthritis scale. Foot Ankle Int 19:466–471
EuroQol G (1990) EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 16:199–208
Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR, Dopirak RM (2008) Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med 36:1750–1762
Georgiannos D, Bisbinas I, Badekas A (2014) Osteochondral transplantation of autologous graft for the treatment of osteochondral lesions of talus: 5- to 7-year follow-up. Knee Surg Sports Traumatol Arthrosc 24:3722–3729
Giannini S, Vannini F (2004) Operative treatment of osteochondral lesions of the talar dome: current concepts review. Foot Ankle Int 25:168–175
Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B (2008) Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med 36:873–880
Giza E, Sullivan M, Ocel D, Lundeen G, Mitchell ME, Veris L, Walton J (2010) Matrix-induced autologous chondrocyte implantation of talus articular defects. Foot Ankle Int 31:747–753
Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR (2012) Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am 94:1105–1110
Halasi T, Kynsburg A, Tallay A, Berkes I (2004) Development of a new activity score for the evaluation of ankle instability. Am J Sports Med 32:899–908
Hangody L, Kish G, Modis L, Szerb I, Gaspar L, Dioszegi Z, Kendik Z (2001) Mosaicplasty for the treatment of osteochondritis dissecans of the talus: two to seven year results in 36 patients. Foot Ankle Int 22:552–558
Hepple S, Winson IG, Glew D (1999) Osteochondral lesions of the talus: a revised classification. Foot Ankle Int 20:789–793
Hintermann B, Wagener J, Knupp M, Schweizer C, Schweizer DJ (2015) Treatment of extended osteochondral lesions of the talus with a free vascularised bone graft from the medial condyle of the femur. Bone Joint J 97:1242–1249
Johanson NA, Liang MH, Daltroy L, Rudicel S, Richmond J (2004) American Academy of Orthopaedic Surgeons lower limb outcomes assessment instruments. Reliability, validity, and sensitivity to change. J Bone Joint Surg Am 86:902–909
Johnson B, Lever C, Roberts S, Richardson J, McCarthy H, Harrison P, Laing P, Makwana N (2013) Cell cultured chondrocyte implantation and scaffold techniques for osteochondral talar lesions. Foot Ankle Clin 18:135–150
Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M (1994) Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int 15:349–353
Kubosch EJ, Erdle B, Izadpanah K, Kubosch D, Uhl M, Sudkamp NP, Niemeyer P (2016) Clinical outcome and T2 assessment following autologous matrix-induced chondrogenesis in osteochondral lesions of the talus. Int Orthop 40:65–71
Lee KT, Kim JS, Young KW, Lee YK, Park YU, Kim YH, Cho HK (2013) The use of fibrin matrix-mixed gel-type autologous chondrocyte implantation in the treatment for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc 21:1251–1260
Loveday D, Clifton R, Robinson A (2010) Interventions for treating osteochondral defects of the talus in adults. Cochrane Database Syst Rev 4:CD008104
Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S (2010) A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med 38:880–890
Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S (2006) Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol 57:16–23
Mazur JM, Schwartz E, Simon SR (1979) Ankle arthrodesis. Long-term follow-up with gait analysis. J Bone Joint Surg Am 61:964–975
McCarty EC, Fader RR, Mitchell JJ, Glenn RE Jr, Potter HG, Spindler KP (2016) Fresh osteochondral allograft versus autograft: twelve-month results in isolated canine knee defects. Am J Sports Med 44:2354–2365
McCollum GA, Myerson MS, Jonck J (2013) Managing the cystic osteochondral defect: allograft or autograft. Foot Ankle Clin 18:113–133
Mintz DN, Tashjian GS, Connell DA, Deland JT, O’Malley M, Potter HG (2003) Osteochondral lesions of the talus: a new magnetic resonance grading system with arthroscopic correlation. Arthroscopy 19:353–359
Moineddin R, Matheson FI, Glazier RH (2007) A simulation study of sample size for multilevel logistic regression models. BMC Med Res Methodol 7:34
Naal FD, Impellizzeri FM, Huber M, Rippstein PF (2008) Cross-cultural adaptation and validation of the Foot Function Index for use in German-speaking patients with foot complaints. Foot Ankle Int 29:1222–1228
Nehrer S, Domayer SE, Hirschfeld C, Stelzeneder D, Trattnig S, Dorotka R (2011) Matrix-associated and autologous chondrocyte transplantation in the ankle: clinical and MRI follow-up after 2 to 11 years. Cartilage 2:81–91
Niemeyer P, Salzmann G, Schmal H, Mayr H, Sudkamp NP (2012) Autologous chondrocyte implantation for the treatment of chondral and osteochondral defects of the talus: a meta-analysis of available evidence. Knee Surg Sports Traumatol Arthrosc 20:1696–1703
O’Driscoll SW (1998) The healing and regeneration of articular cartilage. J Bone Joint Surg Am 80:1795–1812
Ogilvie-Harris DJ, Mahomed N, Demaziere A (1993) Anterior impingement of the ankle treated by arthroscopic removal of bony spurs. J Bone Joint Surg Br 75:437–440
Outerbridge RE (2001) The etiology of chondromalacia patellae. 1961. Clin Orthop Relat Res 5–8
Pomajzl RJ, Baker EA, Baker KC, Fleischer MM, Salisbury MR, Phillips DM, Fortin PT (2016) Case series with histopathologic and radiographic analyses following failure of fresh osteochondral allografts of the talus. Foot Ankle Int 37:958–967
Raikin SM (2009) Fresh osteochondral allografts for large-volume cystic osteochondral defects of the talus. J Bone Joint Surg Am 91:2818–2826
Ramponi L, Yasui Y, Murawski CD, Ferkel RD, DiGiovanni CW, Kerkhoffs GM, Calder JD, Takao M, Vannini F, Choi WJ, Lee JW, Stone J, Kennedy JG (2016) Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med 45:1698–1705
Reilingh ML, van Bergen CJ, Gerards RM, van Eekeren IC, de Haan RJ, Sierevelt IN, Kerkhoffs GM, Krips R, Meuffels DE, van Dijk CN, Blankevoort L (2016) Effects of pulsed electromagnetic fields on return to sports after arthroscopic debridement and microfracture of osteochondral talar defects: a randomized, double-blind, placebo-controlled, multicenter trial. Am J Sports Med 44:1292–1300
Roberts S, McCall IW, Darby AJ, Menage J, Evans H, Harrison PE, Richardson JB (2003) Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther 5:R60–R73
Robinson DE, Winson IG, Harries WJ, Kelly AJ (2003) Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Br 85:989–993
Roos EM, Brandsson S, Karlsson J (2001) Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int 22:788–794
Savva N, Jabur M, Davies M, Saxby T (2007) Osteochondral lesions of the talus: results of repeat arthroscopic debridement. Foot Ankle Int 28:669–673
Thermann H, Driessen A, Becher C (2008) Autologous chondrocyte transplantation in the treatment of articular cartilage lesions of the talus. Orthopade 37:232–239
Valderrabano V, Miska M, Leumann A, Wiewiorski M (2013) Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med 41:519–527
van Bergen CJ, Reilingh ML, van Dijk CN (2011) Tertiary osteochondral defect of the talus treated by a novel contoured metal implant. Knee Surg Sports Traumatol Arthrosc 19:999–1003
van Bergen CJ, van Eekeren IC, Reilingh ML, Sierevelt IN, van Dijk CN (2013) Treatment of osteochondral defects of the talus with a metal resurfacing inlay implant after failed previous surgery: a prospective study. Bone Joint J 95:1650–1655
Ware J Jr, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233
Ware JE Jr, Gandek B, Kosinski M, Aaronson NK, Apolone G, Brazier J, Bullinger M, Kaasa S, Leplege A, Prieto L, Sullivan M, Thunedborg K (1998) The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 51:1167–1170
Wells GA SB, O’Connell D, Peterson J, Welch V, Losos M et al (2016) The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed Jul 2016
Whittaker JP, Smith G, Makwana N, Roberts S, Harrison PE, Laing P, Richardson JB (2005) Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br 87:179–183
Woelfle JV, Reichel H, Javaheripour-Otto K, Nelitz M (2013) Clinical outcome and magnetic resonance imaging after osteochondral autologous transplantation in osteochondritis dissecans of the talus. Foot Ankle Int 34:173–179
Wulker N, Wirth CJ, Rudert M (1996) Treatment of fibular capsular ligament rupture. A multicenter study. Z Orthop Ihre Grenzgeb 134:149–154
Yoon HS, Park YJ, Lee M, Choi WJ, Lee JW (2014) Osteochondral autologous transplantation is superior to repeat arthroscopy for the treatment of osteochondral lesions of the talus after failed primary arthroscopic treatment. Am J Sports Med 42:1896–1903
Zengerink M, Struijs PA, Tol JL, van Dijk CN (2010) Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc 18:238–246
Acknowledgements
The authors would like to thank F. S. van Etten-Jamaludin, clinical librarian, for her help with the search and Dr. R. Holman, statistician, for her help with the statistical analysis. The authors also thank Mr. H. K. Marchbank for proofreading the manuscript.
Author’s contributions
KL and JD both contributed to the conception, design and data collection of the study, performed the analysis and interpretation of all data and wrote the manuscript. MR, CvB, SS and GK contributed to the conception and the design of the review and contributed to the data collection and analysis of the study. MR, CvB, SS and GK also performed a third-party adjudication process and contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
There is no funding source.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
None.
Additional information
K. T. A. Lambers and J. Dahmen both contributed equally to this work and thus share first authorship.
Appendices
Appendix 1
Full electronic search strategy used in this systematic review.
1. PUBMED
# | Searches | Results |
|---|---|---|
1 | (“Osteochondritis Dissecans”[Mesh] OR osteochondritis dissecans[tiab] OR osteochondrosis dissecans[tiab] OR osteochondrolysis[tiab] OR ((osteochondral[tiab] OR chondral[tiab] OR transchondral[tiab] OR cartilage*[tiab]) AND (defect*[tiab] OR lesion*[tiab])) OR OCD[tiab] OR OLT[tiab]) AND (“Talus”[Mesh] OR talus[tiab] OR talar*[tiab] OR ankle[tiab]) | Total number of results 1996–2016: 986 hits |
2. EMBASE (OVID)
# | Searches | Results |
|---|---|---|
1 | (osteochondritis dissecans/or (osteochondritis dissecans or osteochondrosis dissecans or osteochondrolysis or OCD or OLT).ti,ab,kw. or ((osteochondral or chondral or osteochondral or transchondral or cartilage*) adj3 (defect* or lesion*)).ti,ab,kw.) and (talus/or (talus or talar* or ankle).ti,ab,kw.) | 1351 |
2 | limit 1 to yr = ”1996-Current” | 1097 |
3. COCHRANE LIBRARY
# | Searches | Results |
|---|---|---|
1 | MeSH descriptor: [Osteochondritis Dissecans] explode all trees | 8 |
2 | osteochondritis dissecans or osteochondrosis dissecans or osteochondrolysis or OCD or OLT:ti,ab,kw (Word variations have been searched) | 1117 |
3 | (osteochondral or chondral or transchondral or cartilage*) and (defect* or lesion*):ti,ab,kw (Word variations have been searched) | 305 |
4 | #1 or #2 or #3 | 1410 |
5 | MeSH descriptor: [Talus] explode all trees | 27 |
6 | talus or talar* or ankle:ti,ab,kw (Word variations have been searched) | 4866 |
7 | #5 or #6 | 4866 |
8 | #4 and #7, Publication Year from 1996 to 2016, in Cochrane Reviews (Reviews and Protocols), Other Reviews and Trials | 23 |
Appendix 2
Quality Assessment Scale Utilized
NEWCASTLE-OTTAWA QUALITY ASSESSMENT SCALE
NONRANDOMIZED STUDIES ADJUSTED FOR CASE SERIES
Note: A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories.
Study Design
-
1.
Type of Study
-
(a)
Prospective*
-
(b)
Retrospective
-
(c)
Other
-
(d)
Not described
-
(a)
-
2.
Set-up
-
(a)
According to protocol*
-
(b)
Without protocol
-
(c)
No protocol described.
-
(a)
Selection
-
3.
Representativeness of the exposed cohort
-
(a)
Truly representative of the average talar osteochondral defect patient (after failed previous surgery) in the community*
-
(b)
Somewhat representative of the average talar osteochondral defect patient (after failed previous surgery) in the community*
-
(c)
Selected group of patients by orthopaedic surgeon
-
(d)
No description of the derivation of the cohort.
-
(a)
Outcome
-
4.
Assessment of outcome
-
(a)
Independent blind assessment*
-
(b)
Record linkage*
-
(c)
Self-report
-
(d)
No description.
-
(a)
-
5.
Adequacy of follow-up of series
-
(a)
Complete follow-up—all patients accounted for*
-
(b)
Subjects lost to follow-up unlikely to introduce bias—small number lost(<5%)*
-
(c)
Follow-up rate <95% and no description of those lost
-
(d)
No statement.
-
(a)
Number of assigned stars
Study (title, author, year) | Study design | Selection | Outcome |
Each included study was graded on methodological quality by two independent reviewers utilizing adjusted version of the Newcastle–Ottawa scale which is included above. Categories of study design, selection of patients, and outcome were scored by means of a scoring system using quantitive amounts of stars, and, respectively, for each category a maximum of 2 stars 1 star, and 2 stars could be obtained (maximum is 5 stars).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lambers, K.T.A., Dahmen, J., Reilingh, M.L. et al. No superior surgical treatment for secondary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc 26, 2158–2170 (2018). https://doi.org/10.1007/s00167-017-4629-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-017-4629-0